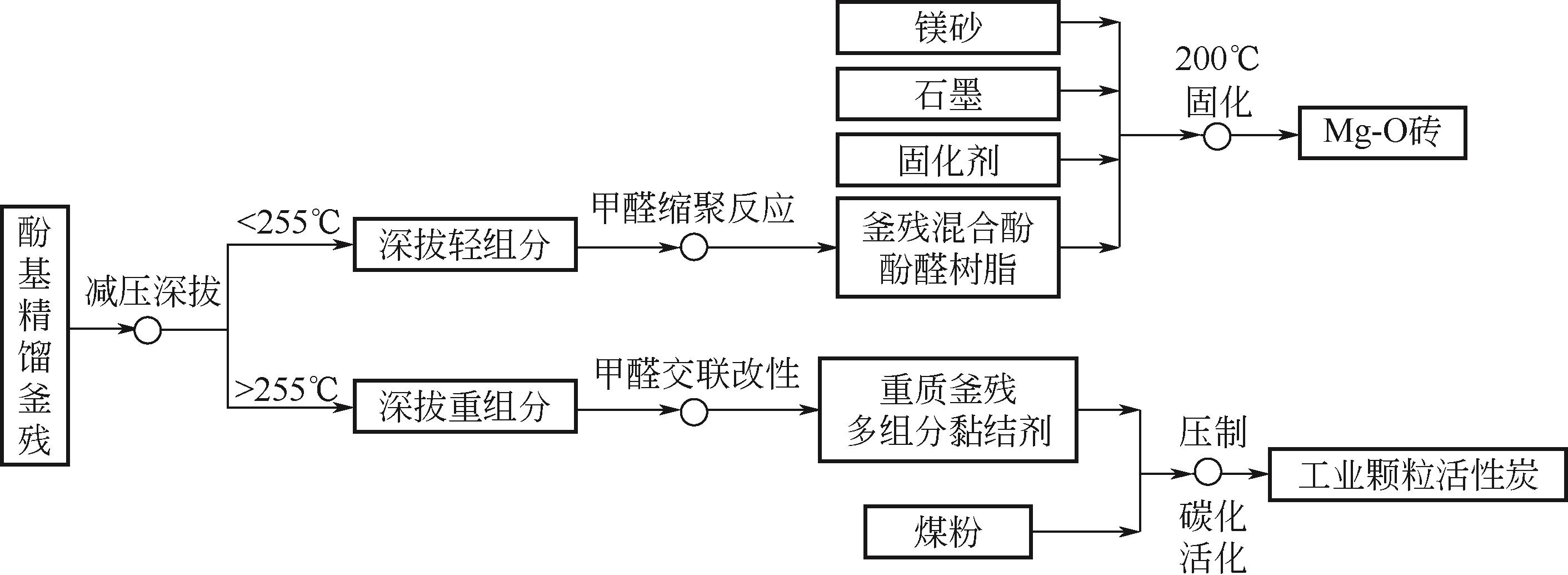
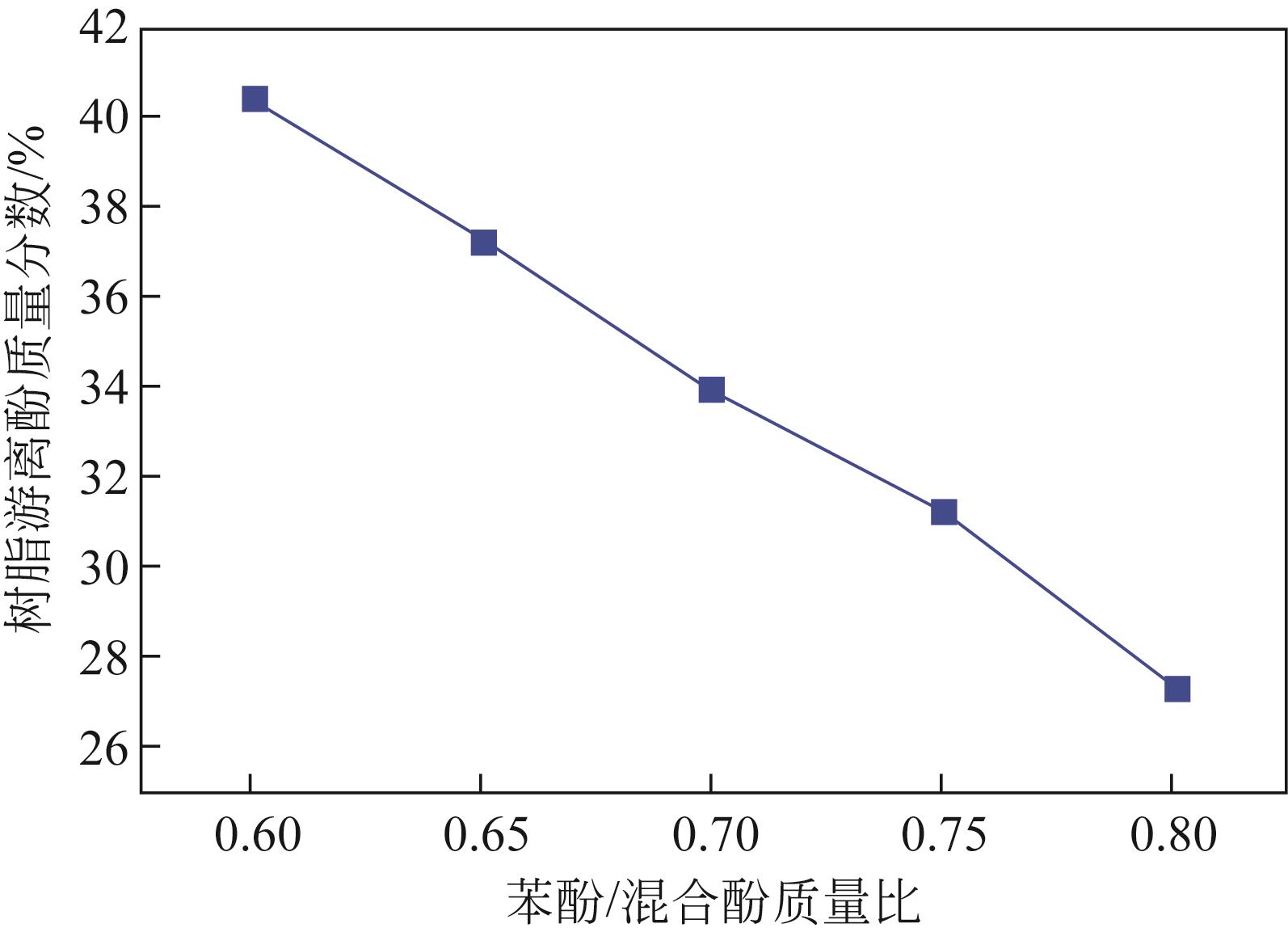
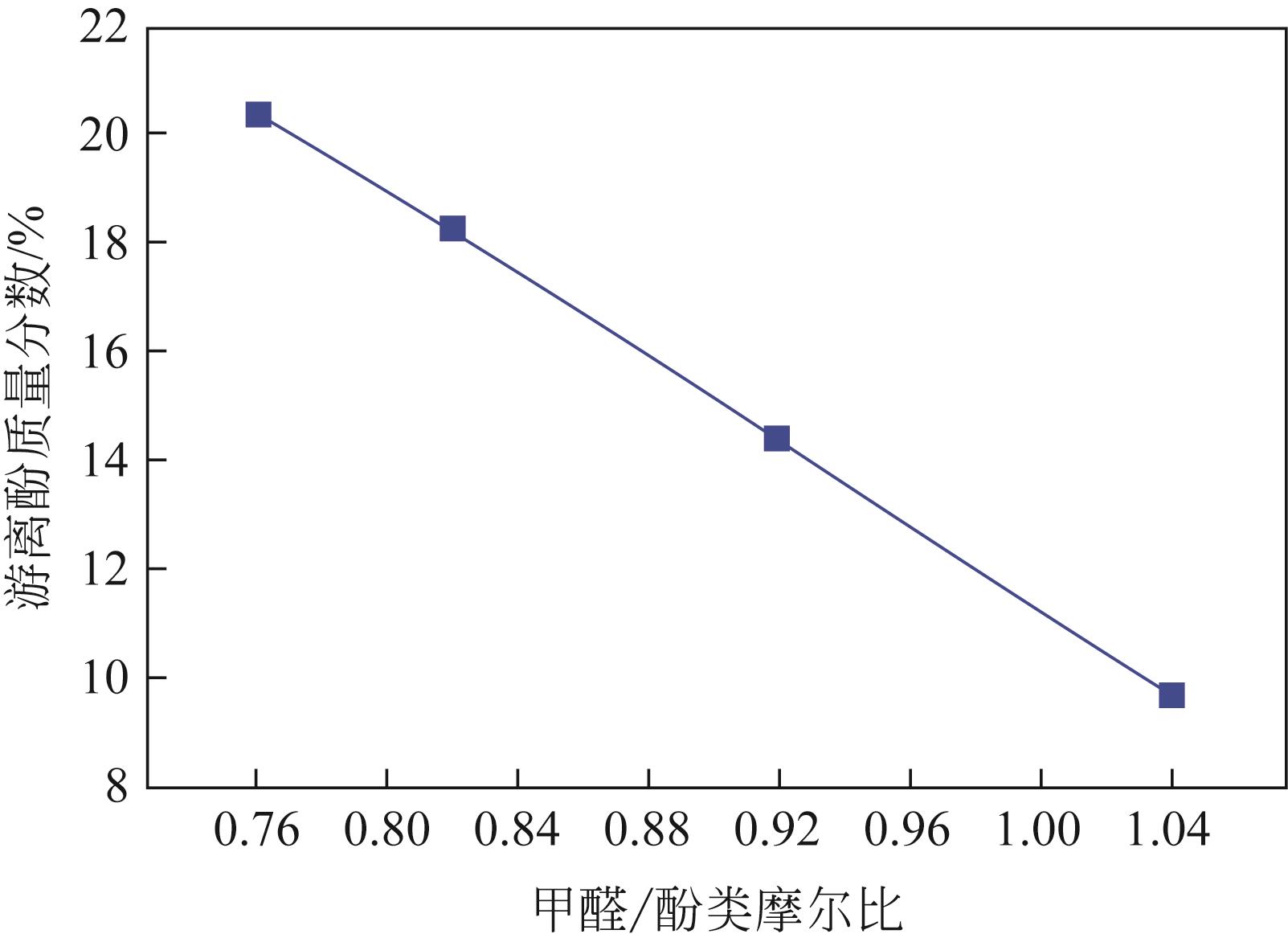
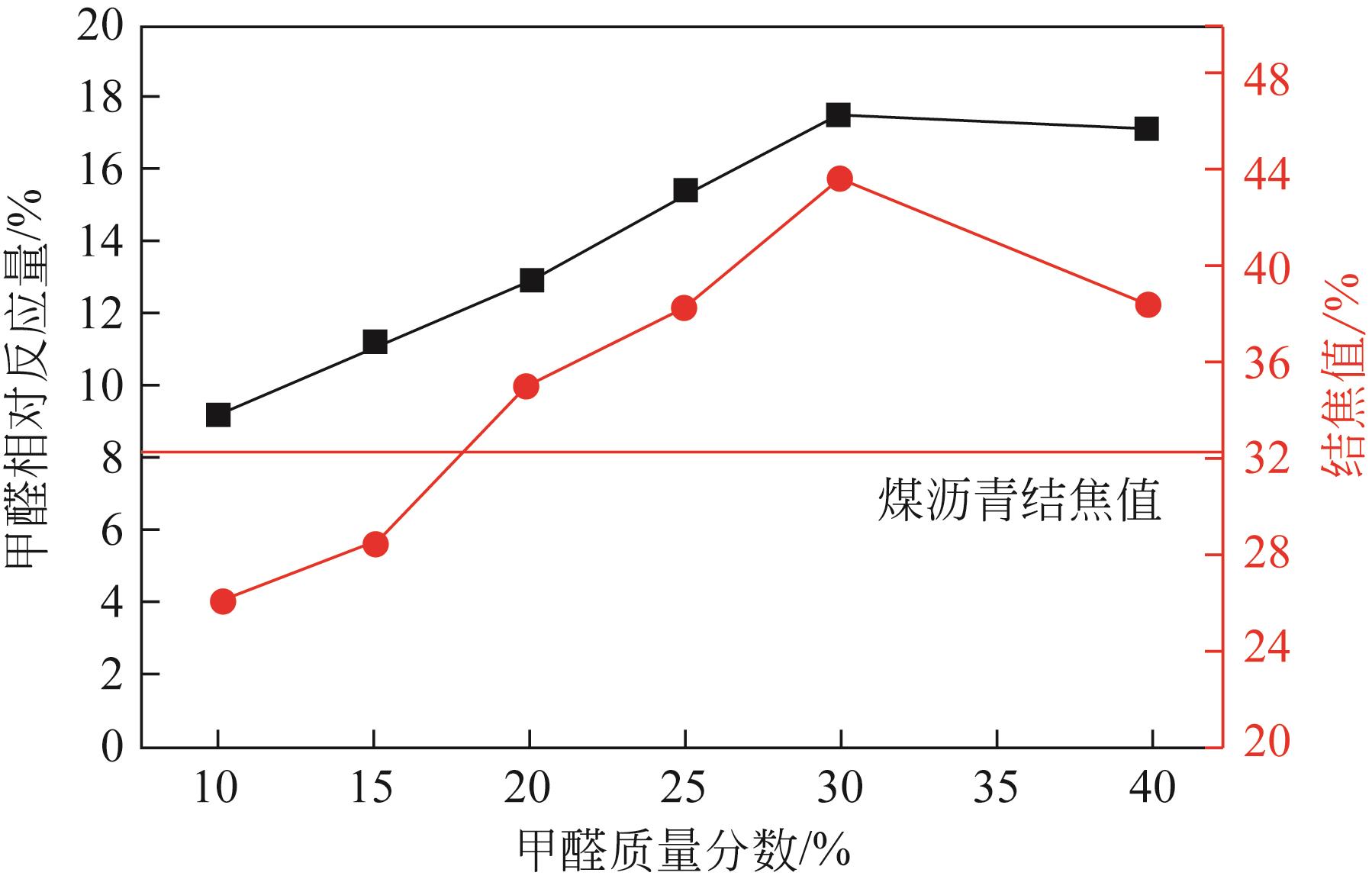
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (6): 3360-3371.DOI: 10.16085/j.issn.1000-6613.2021-1535
• Resources and environmental engineering • Previous Articles Next Articles
Process analysis of resource utilization of phenol-based distillation residue from coal chemical industry
CAI Sichao1,2( ), ZHOU Jing1,2, DU Jinze1,2, LI Fangzhou3, LI Yuansen1,2, HE Lin1,2,3(
), ZHOU Jing1,2, DU Jinze1,2, LI Fangzhou3, LI Yuansen1,2, HE Lin1,2,3( ), LI Xingang1,2,3, WANG Chengyang1,2
), LI Xingang1,2,3, WANG Chengyang1,2
- 1.College of Chemical Engineering, Tianjin University, Tianjin 300072, China
2.National Engineering Research Center of Distillation Technology, Tianjin 300072, China
3.Zhejiang Institute of Tianjin University, Ningbo 315000, Zhejiang, China
-
Received:2021-07-20Revised:2021-10-02Online:2022-06-21Published:2022-06-10 -
Contact:HE Lin
煤化工酚基精馏釜残资源化利用过程初步分析
蔡思超1,2( ), 周静1,2, 杜金泽1,2, 李方舟3, 李源森1,2, 何林1,2,3(
), 周静1,2, 杜金泽1,2, 李方舟3, 李源森1,2, 何林1,2,3( ), 李鑫钢1,2,3, 王成扬1,2
), 李鑫钢1,2,3, 王成扬1,2
- 1.天津大学化工学院,天津 300072
2.精馏技术国家工程研究中心,天津 300072
3.天津大学浙江研究院,浙江 宁波 315000
-
通讯作者:何林 -
作者简介:蔡思超(1998—),男,硕士研究生,研究方向为有机固废资源化与无害化转化。E-mail:sichaocai@tju.edu.cn 。 -
基金资助:国家重点研发计划(2018YFC1902100)
CLC Number:
Cite this article
CAI Sichao, ZHOU Jing, DU Jinze, LI Fangzhou, LI Yuansen, HE Lin, LI Xingang, WANG Chengyang. Process analysis of resource utilization of phenol-based distillation residue from coal chemical industry[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3360-3371.
蔡思超, 周静, 杜金泽, 李方舟, 李源森, 何林, 李鑫钢, 王成扬. 煤化工酚基精馏釜残资源化利用过程初步分析[J]. 化工进展, 2022, 41(6): 3360-3371.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-1535
| 精馏釜残成分 | 河南 | 新疆 | 云南 | |||
|---|---|---|---|---|---|---|
| 0~255℃ | 255~450℃ | 0~250℃ | 250~450℃ | 0~270℃ | 270~550℃ | |
| 苯酚 | 0.85 | 4.04 | 2.80 | 0.71 | 13.96 | 0.96 |
| 单取代基苯酚 | 3.27 | 6.06 | 4.01 | 3.29 | 27.16 | 8.10 |
| 多取代基苯酚 | 6.76 | 3.85 | 5.83 | 6.80 | 14.93 | 4.57 |
| 苯二酚 | 12.80 | 4.36 | 14.18 | 10.10 | 0.88 | 1.78 |
| 单取代基苯二酚 | 32.26 | 21.08 | 39.67 | 37.48 | 7.85 | 15.28 |
| 多取代基苯二酚 | 14.44 | 5.79 | 10.20 | 15.66 | 6.27 | 7.90 |
| 多环酚 | 4.97 | 21.95 | 3.13 | 6.15 | 8.91 | 24.17 |
| 其他芳环类 | 1.67 | 7.88 | 1.77 | 1.36 | 7.69 | 13.61 |
| 酸和酮 | 17.31 | 2.76 | 4.97 | 5.47 | 0.29 | 2.14 |
| 其他 | 5.67 | 22.24 | 13.44 | 12.97 | 12.06 | 21.49 |
| 收率 | 32.75 | 18.35 | 15.60 | 21.14 | 37.87 | 21.86 |
| 精馏釜残成分 | 河南 | 新疆 | 云南 | |||
|---|---|---|---|---|---|---|
| 0~255℃ | 255~450℃ | 0~250℃ | 250~450℃ | 0~270℃ | 270~550℃ | |
| 苯酚 | 0.85 | 4.04 | 2.80 | 0.71 | 13.96 | 0.96 |
| 单取代基苯酚 | 3.27 | 6.06 | 4.01 | 3.29 | 27.16 | 8.10 |
| 多取代基苯酚 | 6.76 | 3.85 | 5.83 | 6.80 | 14.93 | 4.57 |
| 苯二酚 | 12.80 | 4.36 | 14.18 | 10.10 | 0.88 | 1.78 |
| 单取代基苯二酚 | 32.26 | 21.08 | 39.67 | 37.48 | 7.85 | 15.28 |
| 多取代基苯二酚 | 14.44 | 5.79 | 10.20 | 15.66 | 6.27 | 7.90 |
| 多环酚 | 4.97 | 21.95 | 3.13 | 6.15 | 8.91 | 24.17 |
| 其他芳环类 | 1.67 | 7.88 | 1.77 | 1.36 | 7.69 | 13.61 |
| 酸和酮 | 17.31 | 2.76 | 4.97 | 5.47 | 0.29 | 2.14 |
| 其他 | 5.67 | 22.24 | 13.44 | 12.97 | 12.06 | 21.49 |
| 收率 | 32.75 | 18.35 | 15.60 | 21.14 | 37.87 | 21.86 |
| 组分 | MT-LRPF1 | MT-LRPF2 | MT-Nv |
|---|---|---|---|
| 镁砂5~3mm | 11.0 | 11.0 | 11.0 |
| 镁砂3~1mm | 26.0 | 26.0 | 26.0 |
| 镁砂1~0.08mm | 29.0 | 29.0 | 29.0 |
| 镁砂≤80μm | 20.0 | 20.0 | 20.0 |
| 石墨≤300μm | 10.5 | 10.5 | 10.5 |
| 釜残树脂LRPF-1 | 3.5 | — | — |
| 釜残树脂LRPF-2 | — | 3.5 | |
| 商用树脂Nv | — | — | 3.5 |
| 组分 | MT-LRPF1 | MT-LRPF2 | MT-Nv |
|---|---|---|---|
| 镁砂5~3mm | 11.0 | 11.0 | 11.0 |
| 镁砂3~1mm | 26.0 | 26.0 | 26.0 |
| 镁砂1~0.08mm | 29.0 | 29.0 | 29.0 |
| 镁砂≤80μm | 20.0 | 20.0 | 20.0 |
| 石墨≤300μm | 10.5 | 10.5 | 10.5 |
| 釜残树脂LRPF-1 | 3.5 | — | — |
| 釜残树脂LRPF-2 | — | 3.5 | |
| 商用树脂Nv | — | — | 3.5 |
| 无机盐种类 | 新疆 | 云南 |
|---|---|---|
| Na | 5.69 | 26.1 |
| Al | 0.189 | 1.47 |
| Si | 0.765 | 1.46 |
| S | 4.01 | 14.3 |
| K | 0.136 | 0.411 |
| Ca | 0.236 | 0.233 |
| Mn | 0.224 | 0 |
| Fe | 55.5 | 13.0 |
| Ni | 0.0475 | 1.01 |
| Cr | 0.0397 | 1.09 |
| Ti | 0 | 0.323 |
| 无机盐种类 | 新疆 | 云南 |
|---|---|---|
| Na | 5.69 | 26.1 |
| Al | 0.189 | 1.47 |
| Si | 0.765 | 1.46 |
| S | 4.01 | 14.3 |
| K | 0.136 | 0.411 |
| Ca | 0.236 | 0.233 |
| Mn | 0.224 | 0 |
| Fe | 55.5 | 13.0 |
| Ni | 0.0475 | 1.01 |
| Cr | 0.0397 | 1.09 |
| Ti | 0 | 0.323 |
| 项目 | 外观 | 黏度(25℃)/Pa·s | 水分/% | 固体含量/% | 残碳量/% | 游离酚/% | pH |
|---|---|---|---|---|---|---|---|
| YB/T 4131—2005 | 棕黑色透明 | 1~30 | ≤6.5 | ≥75 | ≥40 | ≤12 | 4~7.5 |
| Q/0100SQH 001—2018 | 液体 | 1~30 | ≤8.0 | ≥40.0 | ≥25.0 | ≤14.0 | ≤8.0 |
| LRPF1 | 棕黑色半透明 | 27~31 | ≤2.0 | ≥77 | ≥35 | ≤8.5 | 4~7 |
| LRPF2 | 棕黑色半透明 | 26~30 | ≤3.5 | ≥75 | ≥33 | ≤10 | 4~6 |
| 商用树脂Nv | 红棕色透明 | 30~36 | ≤6.5 | ≥77 | ≥42 | ≤9.0 | 6.8~7.2 |
| 项目 | 外观 | 黏度(25℃)/Pa·s | 水分/% | 固体含量/% | 残碳量/% | 游离酚/% | pH |
|---|---|---|---|---|---|---|---|
| YB/T 4131—2005 | 棕黑色透明 | 1~30 | ≤6.5 | ≥75 | ≥40 | ≤12 | 4~7.5 |
| Q/0100SQH 001—2018 | 液体 | 1~30 | ≤8.0 | ≥40.0 | ≥25.0 | ≤14.0 | ≤8.0 |
| LRPF1 | 棕黑色半透明 | 27~31 | ≤2.0 | ≥77 | ≥35 | ≤8.5 | 4~7 |
| LRPF2 | 棕黑色半透明 | 26~30 | ≤3.5 | ≥75 | ≥33 | ≤10 | 4~6 |
| 商用树脂Nv | 红棕色透明 | 30~36 | ≤6.5 | ≥77 | ≥42 | ≤9.0 | 6.8~7.2 |
| MgO-C砖 | 体积密度/g·cm-3 | 显气孔率/% | 常温耐压强度/MPa |
|---|---|---|---|
| MT-LRPF1 | 2.89±0.02 | 4.2±0.3 | 14.8±0.2 |
| MT-LRPF2 | 2.90±0.03 | 4.1±0.5 | 15.3±0.3 |
| MT-Nv | 2.82±0.02 | 4.4±0.5 | 14.9±0.2 |
| MgO-C砖 | 体积密度/g·cm-3 | 显气孔率/% | 常温耐压强度/MPa |
|---|---|---|---|
| MT-LRPF1 | 2.89±0.02 | 4.2±0.3 | 14.8±0.2 |
| MT-LRPF2 | 2.90±0.03 | 4.1±0.5 | 15.3±0.3 |
| MT-Nv | 2.82±0.02 | 4.4±0.5 | 14.9±0.2 |
| 组分 | 质量分数/% |
|---|---|
| C22H33N6O2 | 36.2 |
| C19H42N3O3 | 22.1 |
| C31H63N12O | 16.3 |
| C29H59N12O | 11.86 |
| C15H34N6NaO2 | 7.31 |
| C15H33N12 | 6.17 |
| 组分 | 质量分数/% |
|---|---|
| C22H33N6O2 | 36.2 |
| C19H42N3O3 | 22.1 |
| C31H63N12O | 16.3 |
| C29H59N12O | 11.86 |
| C15H34N6NaO2 | 7.31 |
| C15H33N12 | 6.17 |
| 黏结剂 | 颗粒炭耐磨强度/% |
|---|---|
| 高温煤沥青 | 98.05 |
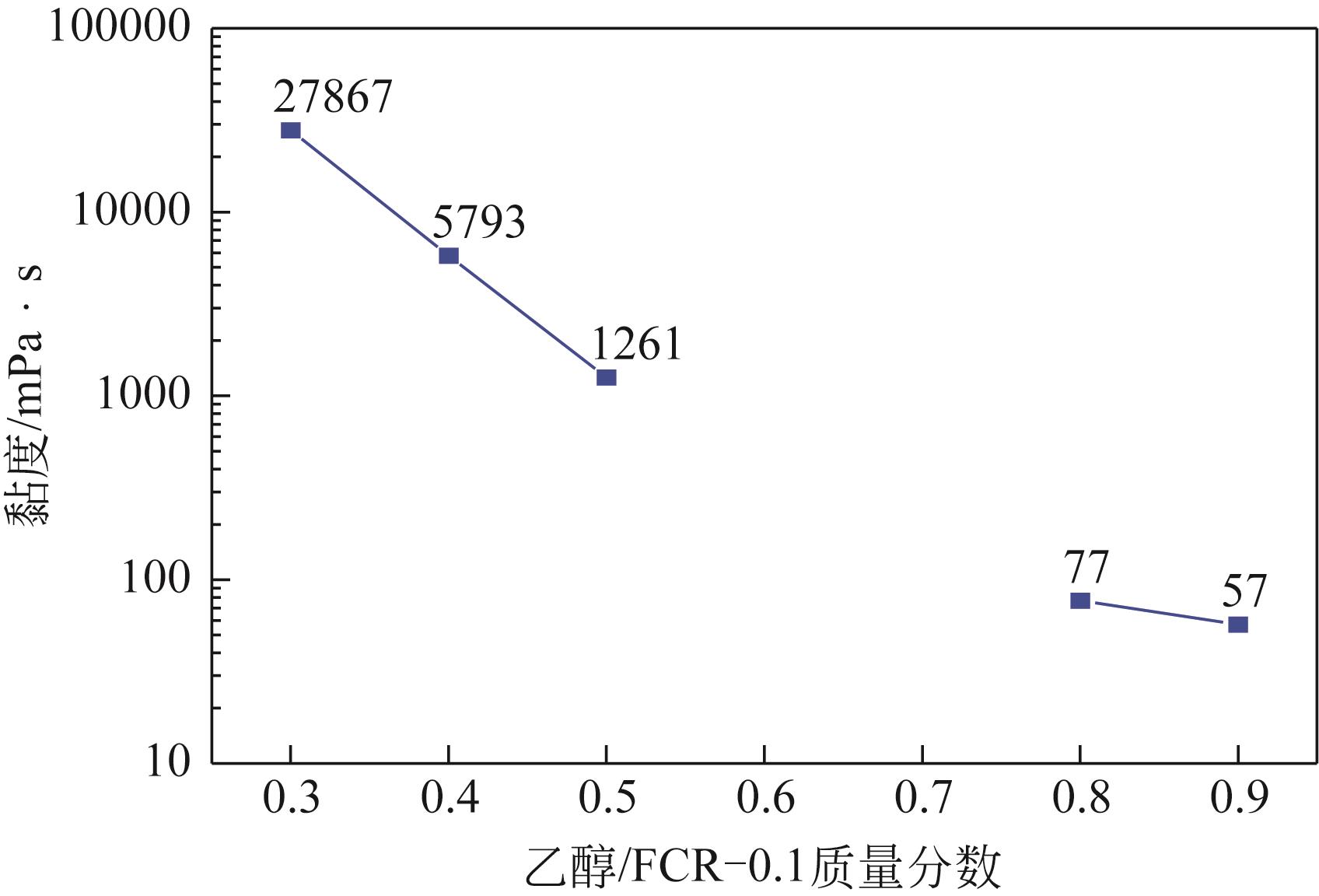
| FCR-0.1 | 98.73 |
| FCR-0.15 | 96.71 |
| 黏结剂 | 颗粒炭耐磨强度/% |
|---|---|
| 高温煤沥青 | 98.05 |
| FCR-0.1 | 98.73 |
| FCR-0.15 | 96.71 |
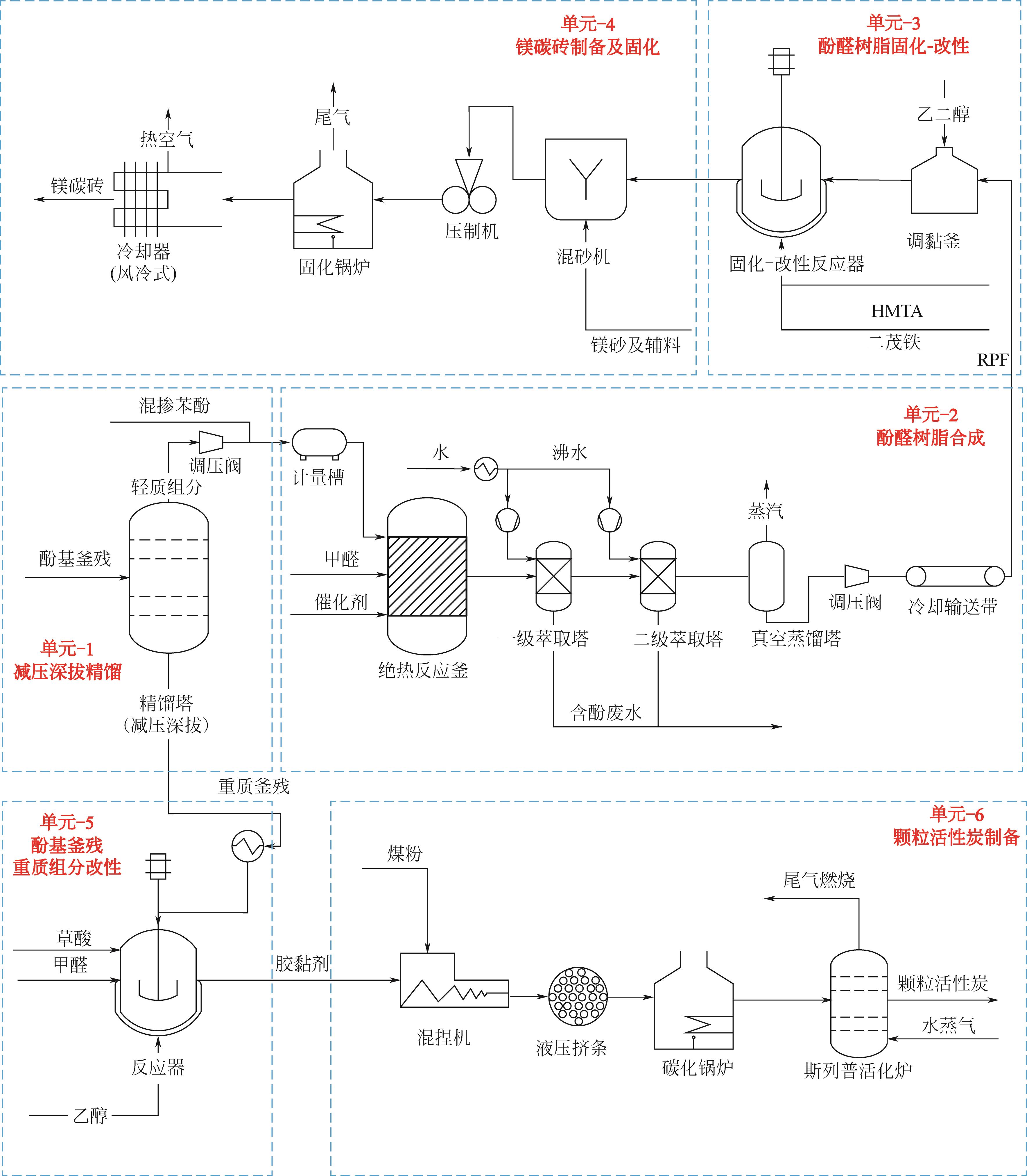
| 单元 | 输入 | 输出 | |||
|---|---|---|---|---|---|
| 物料能耗 | 质量 /kg | 能耗 /MJ | 物料 | 质量 /kg | |
| 单元-1 | 酚基釜残 | 9000 | 轻质酚混合物 | 11790 | |
| 苯酚 | 8842 | 深拔重质组分 | 6052 | ||
| 负荷 | 13112 | ||||
| 单元-2 | 轻质酚混合物 | 11790 | 酚醛树脂 | 14211 | |
| 甲醛溶液 | 7074 | 含酚废水 | 23010 | ||
| 催化剂 | 378 | 蒸汽 | 1263 | ||
| 水 | 19242 | ||||
| 负荷 | 17535 | ||||
| 单元-3 | 酚醛树脂 | 14211 | 耐火材料用树脂 | 18650 | |
| 乙二醇 | 2006 | ||||
| HMTA | 1622 | ||||
| 二茂铁 | 811 | ||||
| 负荷 | 360 | ||||
| 单元-4 | 耐火材料用树脂 | 18650 | 镁碳砖 | 466250 | |
| 镁砂 | 458257 | 废气 | 66607 | ||
| 石墨 | 55950 | ||||
| 负荷 | 35840 | ||||
| 单元-5 | 深拔重质组分 | 6052 | 活性炭黏结剂 | 10979 | |
| 草酸 | 154 | ||||
| 甲醛溶液 | 1636 | ||||
| 乙醇 | 3137 | ||||
| 负荷 | 731 | ||||
| 单元 | 输入 | 输出 | |||
|---|---|---|---|---|---|
| 物料能耗 | 质量 /kg | 能耗 /MJ | 物料 | 质量 /kg | |
| 单元-1 | 酚基釜残 | 9000 | 轻质酚混合物 | 11790 | |
| 苯酚 | 8842 | 深拔重质组分 | 6052 | ||
| 负荷 | 13112 | ||||
| 单元-2 | 轻质酚混合物 | 11790 | 酚醛树脂 | 14211 | |
| 甲醛溶液 | 7074 | 含酚废水 | 23010 | ||
| 催化剂 | 378 | 蒸汽 | 1263 | ||
| 水 | 19242 | ||||
| 负荷 | 17535 | ||||
| 单元-3 | 酚醛树脂 | 14211 | 耐火材料用树脂 | 18650 | |
| 乙二醇 | 2006 | ||||
| HMTA | 1622 | ||||
| 二茂铁 | 811 | ||||
| 负荷 | 360 | ||||
| 单元-4 | 耐火材料用树脂 | 18650 | 镁碳砖 | 466250 | |
| 镁砂 | 458257 | 废气 | 66607 | ||
| 石墨 | 55950 | ||||
| 负荷 | 35840 | ||||
| 单元-5 | 深拔重质组分 | 6052 | 活性炭黏结剂 | 10979 | |
| 草酸 | 154 | ||||
| 甲醛溶液 | 1636 | ||||
| 乙醇 | 3137 | ||||
| 负荷 | 731 | ||||
| 输入 | 价格/CNY·t-1 | 输出 | 价格/CNY·t-1 |
|---|---|---|---|
| 酚基釜残 | 600 | 含酚废水 | -2 |
| 苯酚 | 9300 | 镁碳砖 | 4000 |
| 甲醛溶液 | 1300 | 废气 | -1 |
| 草酸 | 3800 | 活性炭黏结剂 | 3500 |
| 水 | 3 | ||
| 乙二醇 | 5000 | ||
| HMTA | 7000 | ||
| 二茂铁 | 60000 | ||
| 镁砂 | 3000 | ||
| 石墨 | 4000 | ||
甲醛溶液 乙醇 | 1300 7000 |
| 输入 | 价格/CNY·t-1 | 输出 | 价格/CNY·t-1 |
|---|---|---|---|
| 酚基釜残 | 600 | 含酚废水 | -2 |
| 苯酚 | 9300 | 镁碳砖 | 4000 |
| 甲醛溶液 | 1300 | 废气 | -1 |
| 草酸 | 3800 | 活性炭黏结剂 | 3500 |
| 水 | 3 | ||
| 乙二醇 | 5000 | ||
| HMTA | 7000 | ||
| 二茂铁 | 60000 | ||
| 镁砂 | 3000 | ||
| 石墨 | 4000 | ||
甲醛溶液 乙醇 | 1300 7000 |
| 项目 | 费用/×103CNY·d-1 |
|---|---|
| 操作费用 | 1860.88 |
| 原料费用 | 1701.13 |
| 产品销售额 | 1915.87 |
| 公用工程费用 | 0.47022 |
| 设备及安装成本 | 9261.33 |
| 项目 | 费用/×103CNY·d-1 |
|---|---|
| 操作费用 | 1860.88 |
| 原料费用 | 1701.13 |
| 产品销售额 | 1915.87 |
| 公用工程费用 | 0.47022 |
| 设备及安装成本 | 9261.33 |
| 处理方式 | 经济效益/CNY·t-1 |
|---|---|
| 填埋 | -1787 |
| 焚烧 | 829 |
| 热转化综合利用 | 5493 |
| 处理方式 | 经济效益/CNY·t-1 |
|---|---|
| 填埋 | -1787 |
| 焚烧 | 829 |
| 热转化综合利用 | 5493 |
| 1 | DUAN Huabo, HUANG Qifei, WANG Qi, et al. Hazardous waste generation and management in China: a review[J]. Journal of Hazardous Materials, 2008, 158(2/3): 221-227. |
| 2 | 王雄雷, 牛艳霞, 刘刚, 等. 煤焦油渣处理技术的研究进展[J]. 化工进展, 2015, 34(7): 2016-2022. |
| WANG Xionglei, NIU Yanxia, LIU Gang, et al. Research progress of coal tar residue treatment technology[J]. Chemical Industry and Engineering Progress, 2015, 34(7): 2016-2022. | |
| 3 | 蒋学先. 浅论我国危险废物处理处置技术现状[J]. 金属材料与冶金工程, 2009, 37(4): 57-60. |
| JIANG Xuexian. Discussion on the current status of treatment and disposal technology for hazardous waste in our country[J]. Metal Materials and Metallurgy Engineering, 2009, 37(4): 57-60. | |
| 4 | STORM C, RUD¨IGER H, SPLIETHOFF H, et al. Co-pyrolysis of coal/biomass and coal/sewage sludge mixtures[J]. Journal of Engineering for Gas Turbines and Power, 1999, 121(1): 55-63. |
| 5 | MA Yuhui, SU Wei, WANG Qunhui, et al. Discharge and disposal of coking residue and distribution characteristics of PAHs in it[J]. Applied Mechanics and Materials, 2013, 448/449/450/451/452/453: 448-452. |
| 6 | ROCHA E R L, LOPES M S, MACIEL M R W, et al. Recovery and characterization of petroleum residues through the molecular distillation process[J]. Petroleum Science and Technology, 2014, 32(20): 2450-2457. |
| 7 | AN Zhenwei H, KONG Shunli, CHENG Jing, et al. Preparation of efficient carbon-based adsorption material using asphaltenes from asphalt rocks[J]. Industrial & Engineering Chemistry Research, 2019, 58(32): 14785-14794. |
| 8 | 马媛, 杜金泽, 周静, 等. 酚基釜残深拔残渣分析及其热转化制备CO2吸附材料[J]. 化学工业与工程, 2021, 38(1): 43-52. |
| MA Yuan, DU Jinze, ZHOU Jing, et al. Chemical analysis of the phenolic tar residue and its potential conversion to CO2 adsorbents by thermal treatment[J]. Chemical Industry and Engineering, 2021, 38(1): 43-52. | |
| 9 | KA BE T, ISHIHARA A, QIAN E W, et al. Coal and coal-related compounds: structures, reactivity and catalytic reactions[M]. Amsterdam: Elsevier, 2004. |
| 10 | VAEZ I M, PASSANDIDEH-FARD M, MOGHIMAN M, et al. Gasification of heavy fuel oils: a thermochemical equilibrium approach[J]. Fuel, 2011, 90(2): 878-885. |
| 11 | HIR ANO K, ASAMI M. Phenolic resins—100 years of progress and their future[J]. Reactive and Functional Polymers, 2013, 73(2): 256-269. |
| 12 | 黄发荣, 焦杨声. 酚醛树脂及其应用[M]. 北京: 化学工业出版社, 2003. |
| HU ANG Farong, JIAO Yangsheng. Phenolic resin and its application[M]. Beijing: Chemical Industry Press, 2003. | |
| 13 | DEMCHUK Y, SIDUN I, GUNKA V, et al. Effect of phenol-cresol-formaldehyde resin on adhesive and physico-mechanical properties of road bitumen[J]. Chemistry & Chemical Technology, 2018, 12(4): 456-461. |
| 14 | PYSHYE V S, DEMCHUK Y, GUNKA V, et al. Development of mathematical model and identification of optimal conditions to obtain phenol-cresol-formaldehyde resin[J]. Chemistry & Chemical Technology, 2019, 13(2): 212-217. |
| 15 | PAN H, SHUPE T F, HSE C Y. Synthesis and cure kinetics of liquefied wood/phenol/formaldehyde resins[J]. Journal of Applied Polymer Science, 2008, 108(3): 1837-1844. |
| 16 | ZHAO Yong, YAN Ning, FENG M. Synthesis and characterization of bio-based phenol-formaldehyde resol resins from bark autoclave extractives[J]. Forest Products Journal, 2016, 66(1/2): 18-28. |
| 17 | ZHOU Jing, HU Lihong, LIANG Bingchuan, et al. Preparation and characterization of novolac phenol-formaldehyde resins with enzymatic hydrolysis lignin[J]. Journal of the Taiwan Institute of Chemical Engineers, 2015, 54: 178-182. |
| 18 | SOLT P, HERWIJNEN H W G VAN, KONNERTH J. Thermoplastic and moisture-dependent behavior of lignin phenol formaldehyde resins[J]. Journal of Applied Polymer Science, 2019, 136(40): 48011. |
| 19 | YAN Liangcong, CUI Yuhu, GOU Guangjun, et al. Liquefaction of lignin in hot-compressed water to phenolic feedstock for the synthesis of phenol-formaldehyde resins[J]. Composites Part B: Engineering, 2017, 112: 8-14. |
| 20 | CHEN Min, WANG Nan, YU Jingkun, et al. Effect of porosity on carbonation and hydration resistance of CaO materials[J]. Journal of the European Ceramic Society, 2007, 27(4): 1953-1959. |
| 21 | 张碧芳, 王光华. 碳质耐火材料粘结剂的研究[J]. 粘接, 1988, 9(4): 8-12. |
| ZHANG Bifang, WANG Guanghua. Studies on carbon refractory bonding agent[J]. Adhesion in China, 1988, 9(4): 8-12. | |
| 22 | 裘晓鹏, 罗小勇, 陈亮, 等. 危险废物刚性填埋场设计与经济性分析[J]. 环境卫生工程, 2020, 28(6): 80-85. |
| QIU Xiaopeng, LUO Xiaoyong, CHEN Liang, et al. Design and economic analysis of rigid landfill for hazardous waste[J]. Environmental Sanitation Engineering, 2020, 28(6): 80-85. | |
| 23 | 李庆辉. 药业公司危险废物资源化处理工艺方案设计[D]. 济南: 山东大学, 2016. |
| LI Qinghui. Process design for treatment of hazardous waster from a pharmaceutical company[D]. Jinan: Shandong University, 2016. |
| [1] | SHAO Boshi, TAN Hongbo. Simulation on the enhancement of cryogenic removal of volatile organic compounds by sawtooth plate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 84-93. |
| [2] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [3] | ZHANG Yaojie, ZHANG Chuanxiang, SUN Yue, ZENG Huihui, JIA Jianbo, JIANG Zhendong. Application of coal-based graphene quantum dots in supercapacitors [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4340-4350. |
| [4] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [5] | XIU Haoran, WANG Yungang, BAI Yanyuan, ZOU Li, LIU Yang. Combustion characteristics and ash melting behavior of Zhundong coal/municipal sludge blended combustion [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3242-3252. |
| [6] | YANG Hongmei, GAO Tao, YU Tao, QU Chengtun, GAO Jiapeng. Treatment of refractory organics sulfonated phenolic resin with ferrate [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3302-3308. |
| [7] | LI Wenxiu, YANG Yuhang, HUANG Yan, WANG Tao, WANG Lei, FANG Mengxiang. Preparation of ultrafine calcium carbonate by CO2 mineralization using high calcium-based solid waste [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2047-2057. |
| [8] | YANG Ziqiang, LI Fenghai, GUO Weijie, MA Mingjie, ZHAO Wei. Review on phosphorus migration and transformation during municipal sewage sludge heat treatment [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2081-2090. |
| [9] | XING Xianjun, LUO Tian, BU Yuzheng, MA Peiyong. Preparation of biochar from walnut shells activated by H3PO4 and its application in Cr(Ⅵ) adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1527-1539. |
| [10] | LIU Yajuan. Research status of membrane fouling mitigation by PAC in submerged PAC-AMBRs [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 457-468. |
| [11] | LIU Nan, HU Yiming, YANG Ying, LI Hongjin, GAO Zhuqing, HAO Xiuli. Microwave assisted co-pyrolysis of waste polypropylene /activated carbon to produce combustible pyrolysis gas and light pyrolysis oil [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 150-159. |
| [12] | CHEN Xiaoyun, GUO Yadong, DI Lu, BI Dongmei, LI Kaikai, LIN Xiaona. Catalytic co-pyrolysis of biomass and plastic for aromatics production with boron doped activated carbon catalyst [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 199-209. |
| [13] | LIU Dachen, DU Minghui, WANG Heng. Bromination modification of phenolic hydroxyl sites of crosslinked teroctyl phenolic resin [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 382-388. |
| [14] | ZHANG Xinhai, ZHAO Sichen, ZHU Hui, WANG Kai, ZHANG Shoushi. Application of activated carbon fiber supported desulfurizer in mine gas environment [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 415-423. |
| [15] | ZHANG Xinhai, ZHAO Sichen, ZHU Hui, ZHANG Shoushi, WANG Kai. Comparative study on desulfurization performance of various carbon materials combined with sodium carbonate [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 424-435. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||