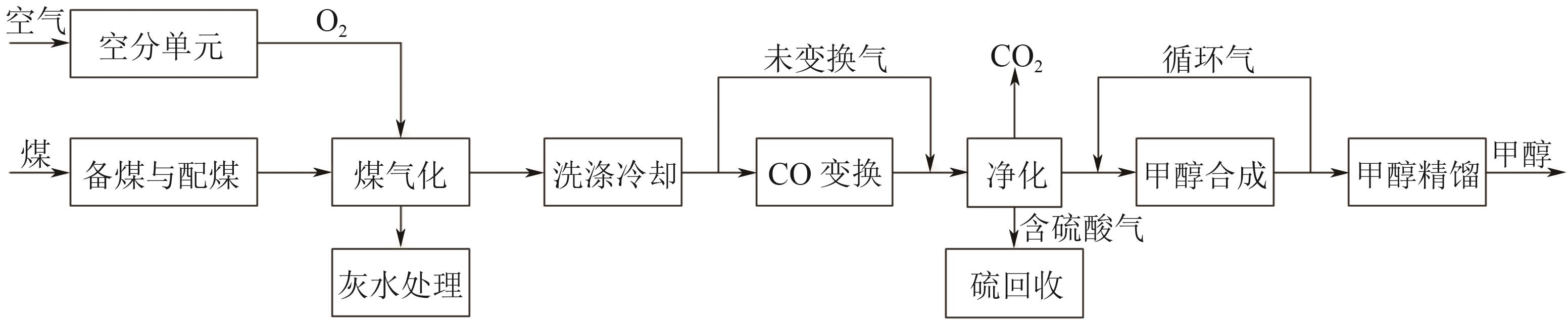
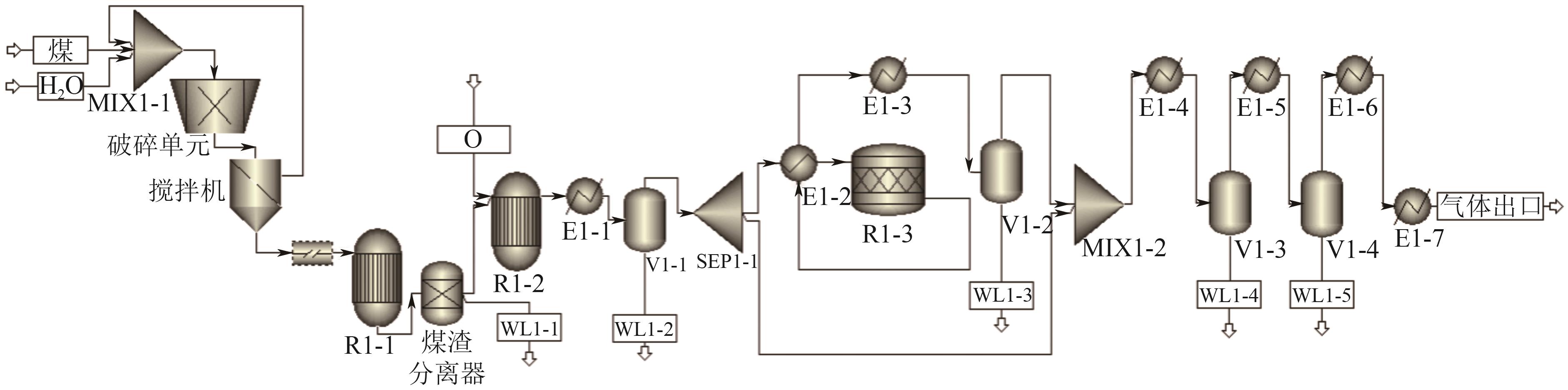
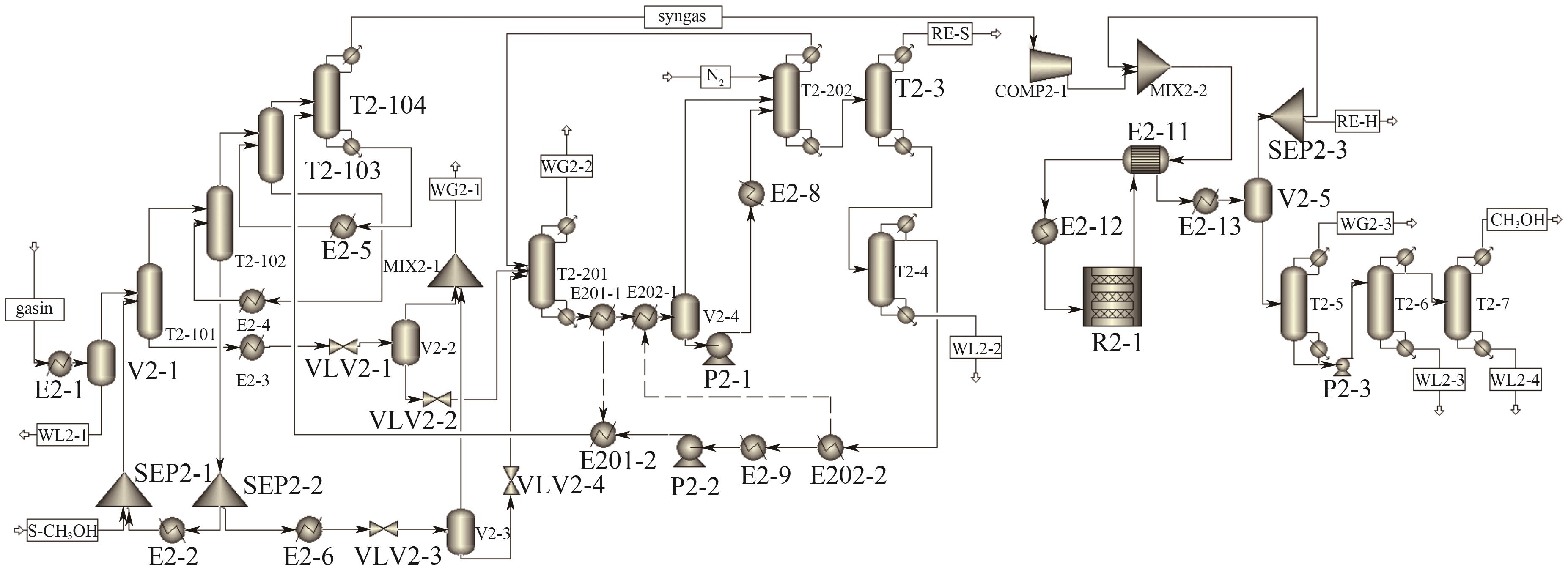
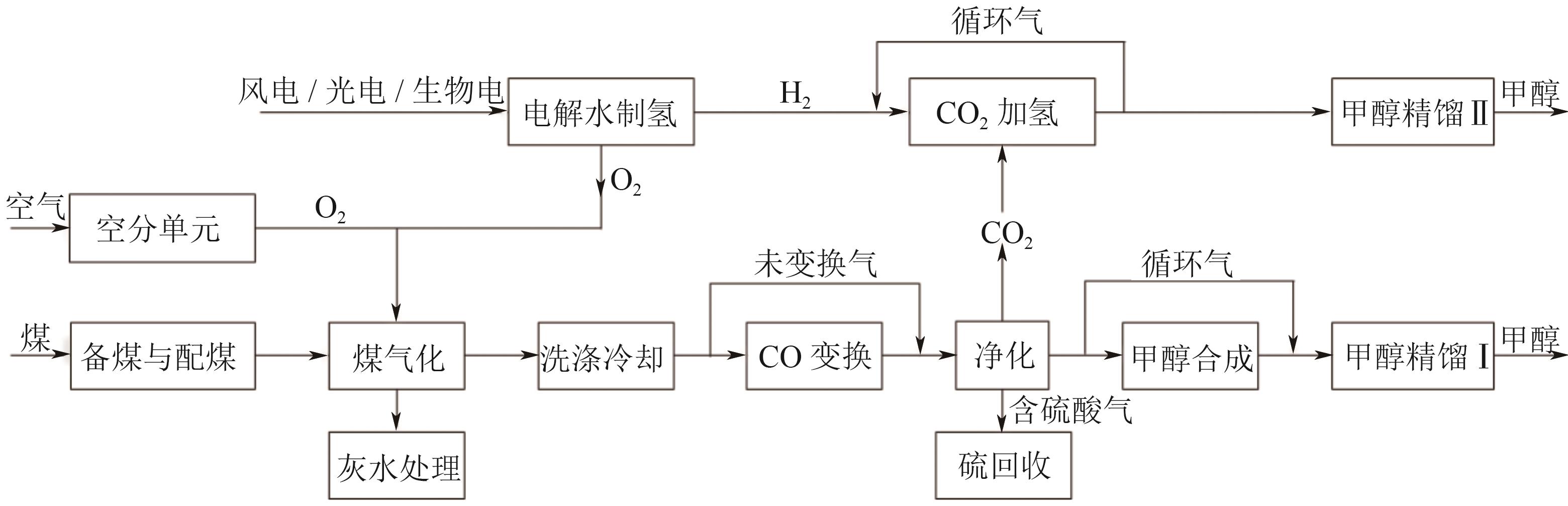
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (8): 4657-4668.DOI: 10.16085/j.issn.1000-6613.2024-1669
• Process systems modeling and simulation • Previous Articles
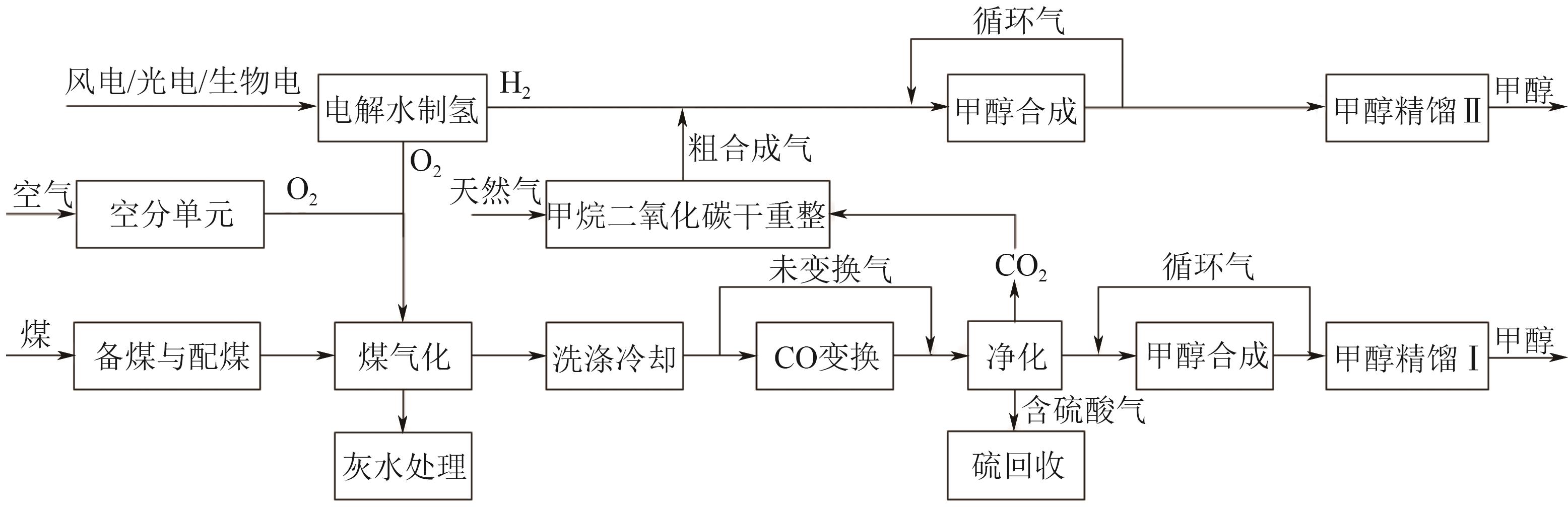
Simulation and techno-economic analysis of new efficient coupling processes between coal to methanol and green hydrogen
YANG Jiacong( ), CHENG Guangxu(
), CHENG Guangxu( ), JIA Tonghua, JIANG Zhao
), JIA Tonghua, JIANG Zhao
- School of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2024-10-17Revised:2025-02-12Online:2025-09-08Published:2025-08-25 -
Contact:CHENG Guangxu
煤制甲醇与绿氢高效耦合新工艺模拟及技术经济分析
- 西安交通大学化学工程与技术学院,陕西 西安 710049
-
通讯作者:程光旭 -
作者简介:杨嘉聪(2000—),男,博士研究生,研究方向为能源化工节能技术。E-mail:yjc18571341687@stu.xjtu.edu.cn。 -
基金资助:陕煤-秦岭计划(SMYJY20210296)
CLC Number:
Cite this article
YANG Jiacong, CHENG Guangxu, JIA Tonghua, JIANG Zhao. Simulation and techno-economic analysis of new efficient coupling processes between coal to methanol and green hydrogen[J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4657-4668.
杨嘉聪, 程光旭, 贾彤华, 姜召. 煤制甲醇与绿氢高效耦合新工艺模拟及技术经济分析[J]. 化工进展, 2025, 44(8): 4657-4668.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1669
| 工业分析 | 质量分数/% | 元素分析 | 质量分数/% | 硫分析 | 质量分数/% |
|---|---|---|---|---|---|
| 水分 | 7.67 | C | 73.67 | 硫化矿硫 | 30 |
| 固定碳(FC) | 56.33 | H | 6.41 | 硫酸盐硫 | 30 |
| 挥发分(VM) | 34.66 | N | 1.15 | 有机硫 | 40 |
| 灰分(ASH) | 9.01 | S | 0.36 | ||
| O | 9.4 |
| 工业分析 | 质量分数/% | 元素分析 | 质量分数/% | 硫分析 | 质量分数/% |
|---|---|---|---|---|---|
| 水分 | 7.67 | C | 73.67 | 硫化矿硫 | 30 |
| 固定碳(FC) | 56.33 | H | 6.41 | 硫酸盐硫 | 30 |
| 挥发分(VM) | 34.66 | N | 1.15 | 有机硫 | 40 |
| 灰分(ASH) | 9.01 | S | 0.36 | ||
| O | 9.4 |
| 工段 | 设备 | 塔板数 | 进料塔板 | 操作压力/kPa |
|---|---|---|---|---|
| 低温甲醇洗 | T2-101 | 15 | 14 | 2830 |
| T2-102 | 3 | 3 | 2810 | |
| T2-103 | 10 | 10 | 2800 | |
| T2-104 | 8 | 8 | 2780 | |
| T2-201 | 40 | 1 | 180 | |
| T2-202 | 15 | 10 | 210 | |
甲醇精馏 (传统工艺) | T2-3 | 12 | 7 | 100 |
| T2-4 | 15 | 1 | 119 | |
| T2-5 | 15 | 10 | 180 | |
| T2-6 | 24 | 20 | 600 | |
| T2-7 | 27 | 20 | 102 | |
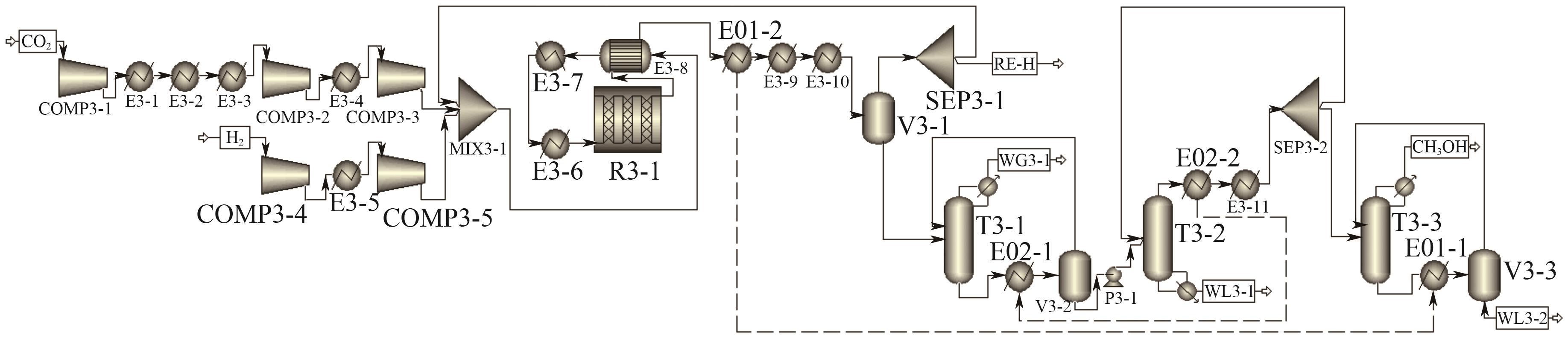
| 新工艺Ⅰ | T3-1 | 10 | 7 | 180 |
| T3-2 | 25 | 21 | 600 | |
| T3-3 | 30 | 23 | 102 | |
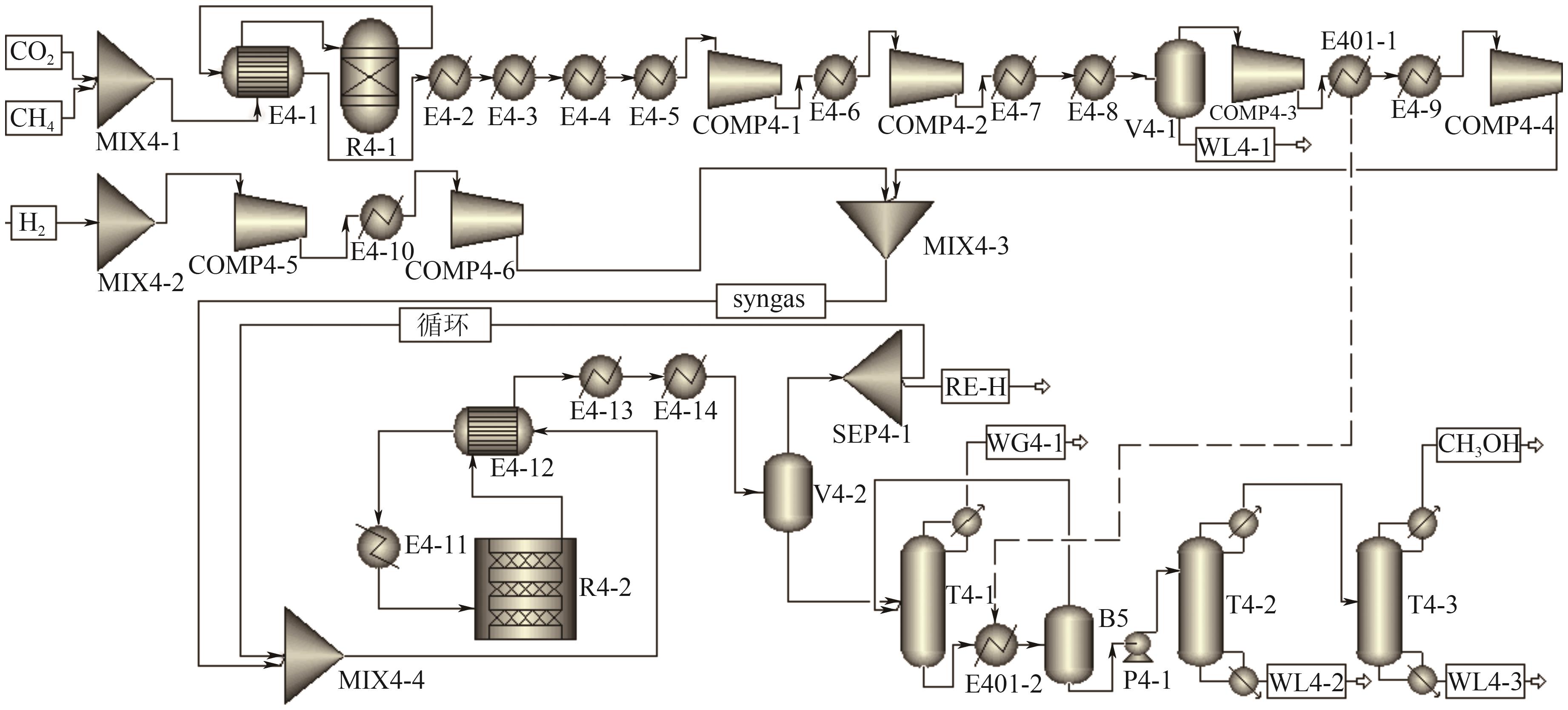
| 新工艺Ⅱ | T4-1 | 11 | 6 | 180 |
| T4-2 | 22 | 13 | 600 | |
| T4-3 | 20 | 10 | 102 |
| 工段 | 设备 | 塔板数 | 进料塔板 | 操作压力/kPa |
|---|---|---|---|---|
| 低温甲醇洗 | T2-101 | 15 | 14 | 2830 |
| T2-102 | 3 | 3 | 2810 | |
| T2-103 | 10 | 10 | 2800 | |
| T2-104 | 8 | 8 | 2780 | |
| T2-201 | 40 | 1 | 180 | |
| T2-202 | 15 | 10 | 210 | |
甲醇精馏 (传统工艺) | T2-3 | 12 | 7 | 100 |
| T2-4 | 15 | 1 | 119 | |
| T2-5 | 15 | 10 | 180 | |
| T2-6 | 24 | 20 | 600 | |
| T2-7 | 27 | 20 | 102 | |
| 新工艺Ⅰ | T3-1 | 10 | 7 | 180 |
| T3-2 | 25 | 21 | 600 | |
| T3-3 | 30 | 23 | 102 | |
| 新工艺Ⅱ | T4-1 | 11 | 6 | 180 |
| T4-2 | 22 | 13 | 600 | |
| T4-3 | 20 | 10 | 102 |
| 项目 | 摩尔分数/% | 温度/℃ | |||
|---|---|---|---|---|---|
| H2 | CO2 | CO | CH4 | ||
| 模拟结果(干基) | 33.19 | 19.89 | 46.25 | 0.01 | 1300 |
| 文献结果(干基) | 34.09 | 20.14 | 45.31 | 0.02 | 1300 |
| 生产数据(干基) | 34.14 | 20.25 | 45.53 | 0.07 | 1314 |
| 项目 | 摩尔分数/% | 温度/℃ | |||
|---|---|---|---|---|---|
| H2 | CO2 | CO | CH4 | ||
| 模拟结果(干基) | 33.19 | 19.89 | 46.25 | 0.01 | 1300 |
| 文献结果(干基) | 34.09 | 20.14 | 45.31 | 0.02 | 1300 |
| 生产数据(干基) | 34.14 | 20.25 | 45.53 | 0.07 | 1314 |
| 流程 | 摩尔分数/% | |||||||
|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | CO | N2 | H2O | COS | H2S | CH4 | |
| 气化后 | 23.56 | 14.12 | 32.83 | 0.37 | 29.02 | 45.16×10-4 | 854.84×10-4 | 0.01 |
| 变换后 | 38.61 | 29.16 | 17.79 | 0.37 | 13.97 | 45.16×10-4 | 854.84×10-4 | 0.01 |
| 净化后 | 66.91 | 1.68 | 30.73 | 0.63 | 0 | 0.01×10-4 | 0.05×10-4 | 0.02 |
| 流程 | 摩尔分数/% | |||||||
|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | CO | N2 | H2O | COS | H2S | CH4 | |
| 气化后 | 23.56 | 14.12 | 32.83 | 0.37 | 29.02 | 45.16×10-4 | 854.84×10-4 | 0.01 |
| 变换后 | 38.61 | 29.16 | 17.79 | 0.37 | 13.97 | 45.16×10-4 | 854.84×10-4 | 0.01 |
| 净化后 | 66.91 | 1.68 | 30.73 | 0.63 | 0 | 0.01×10-4 | 0.05×10-4 | 0.02 |
| 流股 | 组分摩尔分数/% | 摩尔流量 /kmol·h-1 | 质量流量 /kg·h-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | H2O | N2 | CH4 | H2S | COS | CH3OH | |||
| gasin | 38.61 | 17.79 | 29.16 | 13.97 | 0.37 | 0.01 | 854.84×10-4 | 45.16×10-4 | 0 | 6435.63 | 136747.37 |
| WG2-1 | 24.00 | 29.73 | 45.23 | 0 | 0.96 | 0.04 | 297.26×10-4 | 2.74×10-4 | 0.01 | 9.77 | 283.45 |
| WG2-2 | 0.02 | 0.10 | 99.27 | 0 | 0.60 | 0 | 1.42×10-4 | 1.40×10-4 | 0.01 | 1692.25 | 74270.72 |
| WG2-3 | 22.04 | 6.15 | 32.88 | 0 | 11.23 | 1.61 | 598.52×10-4 | 1.48×10-4 | 26.02 | 29.80 | 846.60 |
| WL2-1 | 0 | 0 | 0 | 100.00 | 0 | 0 | 0 | 0 | 0 | 898.95 | 16196.88 |
| WL2-2 | 0 | 0 | 0 | 100.00 | 0 | 0 | 0 | 0 | 0 | 1.50 | 27.02 |
| WL2-3 | 0 | 0 | 0 | 99.67 | 0 | 0 | 0 | 0 | 0.33 | 48.90 | 883.22 |
| WL2-4 | 0 | 0 | 0 | 51.19 | 0 | 0 | 0 | 0 | 48.81 | 3.16 | 78.78 |
| N2 | 0 | 0 | 0 | 0 | 100.00 | 0 | 0 | 0 | 0 | 10 | 280.13 |
| RE-S | 0 | 0 | 79.48 | 0 | 0.04 | 0 | 3.17 | 0.17 | 17.14 | 163.49 | 6812.07 |
| RE-H | 67.10 | 9.35 | 2.33 | 0 | 20.64 | 0.27 | 6.29×10-4 | 0.37×10-4 | 0.31 | 97.93 | 1069.57 |
| CH3OH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100.00 | 1170.44 | 37502.80 |
| 流股 | 组分摩尔分数/% | 摩尔流量 /kmol·h-1 | 质量流量 /kg·h-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | H2O | N2 | CH4 | H2S | COS | CH3OH | |||
| gasin | 38.61 | 17.79 | 29.16 | 13.97 | 0.37 | 0.01 | 854.84×10-4 | 45.16×10-4 | 0 | 6435.63 | 136747.37 |
| WG2-1 | 24.00 | 29.73 | 45.23 | 0 | 0.96 | 0.04 | 297.26×10-4 | 2.74×10-4 | 0.01 | 9.77 | 283.45 |
| WG2-2 | 0.02 | 0.10 | 99.27 | 0 | 0.60 | 0 | 1.42×10-4 | 1.40×10-4 | 0.01 | 1692.25 | 74270.72 |
| WG2-3 | 22.04 | 6.15 | 32.88 | 0 | 11.23 | 1.61 | 598.52×10-4 | 1.48×10-4 | 26.02 | 29.80 | 846.60 |
| WL2-1 | 0 | 0 | 0 | 100.00 | 0 | 0 | 0 | 0 | 0 | 898.95 | 16196.88 |
| WL2-2 | 0 | 0 | 0 | 100.00 | 0 | 0 | 0 | 0 | 0 | 1.50 | 27.02 |
| WL2-3 | 0 | 0 | 0 | 99.67 | 0 | 0 | 0 | 0 | 0.33 | 48.90 | 883.22 |
| WL2-4 | 0 | 0 | 0 | 51.19 | 0 | 0 | 0 | 0 | 48.81 | 3.16 | 78.78 |
| N2 | 0 | 0 | 0 | 0 | 100.00 | 0 | 0 | 0 | 0 | 10 | 280.13 |
| RE-S | 0 | 0 | 79.48 | 0 | 0.04 | 0 | 3.17 | 0.17 | 17.14 | 163.49 | 6812.07 |
| RE-H | 67.10 | 9.35 | 2.33 | 0 | 20.64 | 0.27 | 6.29×10-4 | 0.37×10-4 | 0.31 | 97.93 | 1069.57 |
| CH3OH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100.00 | 1170.44 | 37502.80 |
| 流股 | 组分摩尔分数/% | 摩尔流量 /kmol·h-1 | 质量流量 /kg·h-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | H2O | N2 | CH4 | H2S | COS | CH3OH | |||
| H2 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4698.26 | 9471.13 |
| CO2 | 0.02 | 0.10 | 99.27 | 0 | 0.60 | 0 | 1.42×10-4 | 1.40×10-4 | 0.01 | 1692.25 | 74270.72 |
| RE-H | 72.44 | 1.98 | 23.86 | 0.04 | 1.52 | 0 | 2.61×10-4 | 0.16×10-4 | 0.16 | 660.591 | 8588.21 |
| WG3-1 | 6.56 | 0.30 | 92.93 | 0 | 0.18 | 0.01 | 188.29×10-4 | 11.71×10-4 | 0 | 118.791 | 4891.22 |
| WL3-1 | 0 | 0 | 0 | 99.99 | 0 | 0 | 0 | 0 | 0.01 | 1334.74 | 24048.24 |
| WL3-2 | 0 | 0 | 0 | 84.53 | 0 | 0 | 0 | 0 | 15.47 | 91.11 | 1839.02 |
| CH3OH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100.00 | 1384.66 | 44366.22 |
| 流股 | 组分摩尔分数/% | 摩尔流量 /kmol·h-1 | 质量流量 /kg·h-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | H2O | N2 | CH4 | H2S | COS | CH3OH | |||
| H2 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4698.26 | 9471.13 |
| CO2 | 0.02 | 0.10 | 99.27 | 0 | 0.60 | 0 | 1.42×10-4 | 1.40×10-4 | 0.01 | 1692.25 | 74270.72 |
| RE-H | 72.44 | 1.98 | 23.86 | 0.04 | 1.52 | 0 | 2.61×10-4 | 0.16×10-4 | 0.16 | 660.591 | 8588.21 |
| WG3-1 | 6.56 | 0.30 | 92.93 | 0 | 0.18 | 0.01 | 188.29×10-4 | 11.71×10-4 | 0 | 118.791 | 4891.22 |
| WL3-1 | 0 | 0 | 0 | 99.99 | 0 | 0 | 0 | 0 | 0.01 | 1334.74 | 24048.24 |
| WL3-2 | 0 | 0 | 0 | 84.53 | 0 | 0 | 0 | 0 | 15.47 | 91.11 | 1839.02 |
| CH3OH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100.00 | 1384.66 | 44366.22 |
| 流股 | 组分摩尔分数/% | 摩尔流量 /kmol·h-1 | 质量流量 /kg·h-1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | H2O | N2 | CH4 | H2S | COS | C2H6 | C3H8 | CH3OH | |||
| CO2 | 0.02 | 0.10 | 99.27 | 0 | 0.60 | 0 | 1.42×10-4 | 1.40×10-4 | 0 | 0 | 0.01 | 799.36 | 35082.89 |
| CH4 | 0 | 0 | 0 | 0 | 4.64 | 94.70 | 0 | 0 | 0.55 | 0.11 | 0 | 761.78 | 12729.67 |
| H2 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1581.75 | 3188.61 |
| WL4-1 | 0 | 0 | 0 | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 35.81 | 645.27 |
| WL4-2 | 0 | 0 | 0 | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30.34 | 546.69 |
| WL4-3 | 0 | 0 | 0 | 94.62 | 0 | 0 | 0 | 0 | 0 | 0 | 5.38 | 3.26 | 61.29 |
| RE-H | 45.16 | 22.72 | 5.19 | 0 | 16.10 | 10.53 | 2.92×10-4 | 0 | 0 | 0 | 0.30 | 227.92 | 3613.37 |
| WG4-1 | 8.54 | 8.65 | 42.03 | 0 | 5.08 | 35.68 | 0.02 | 1.13×10-4 | 0 | 0 | 0 | 62.36 | 1761.64 |
| CH3OH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 99.99 | 1384.40 | 44358.73 |
| 流股 | 组分摩尔分数/% | 摩尔流量 /kmol·h-1 | 质量流量 /kg·h-1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | H2O | N2 | CH4 | H2S | COS | C2H6 | C3H8 | CH3OH | |||
| CO2 | 0.02 | 0.10 | 99.27 | 0 | 0.60 | 0 | 1.42×10-4 | 1.40×10-4 | 0 | 0 | 0.01 | 799.36 | 35082.89 |
| CH4 | 0 | 0 | 0 | 0 | 4.64 | 94.70 | 0 | 0 | 0.55 | 0.11 | 0 | 761.78 | 12729.67 |
| H2 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1581.75 | 3188.61 |
| WL4-1 | 0 | 0 | 0 | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 35.81 | 645.27 |
| WL4-2 | 0 | 0 | 0 | 100.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30.34 | 546.69 |
| WL4-3 | 0 | 0 | 0 | 94.62 | 0 | 0 | 0 | 0 | 0 | 0 | 5.38 | 3.26 | 61.29 |
| RE-H | 45.16 | 22.72 | 5.19 | 0 | 16.10 | 10.53 | 2.92×10-4 | 0 | 0 | 0 | 0.30 | 227.92 | 3613.37 |
| WG4-1 | 8.54 | 8.65 | 42.03 | 0 | 5.08 | 35.68 | 0.02 | 1.13×10-4 | 0 | 0 | 0 | 62.36 | 1761.64 |
| CH3OH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 99.99 | 1384.40 | 44358.73 |
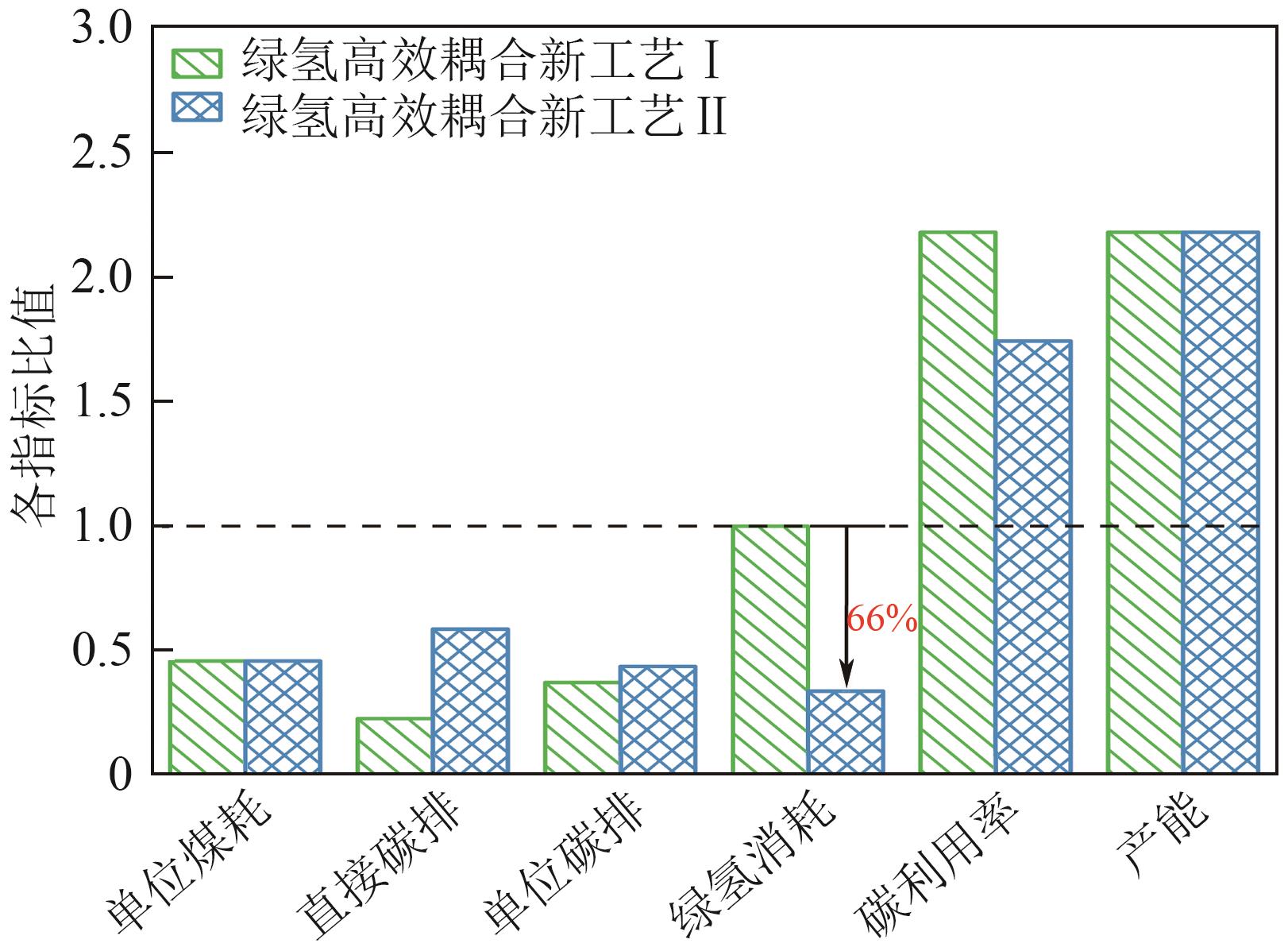
| 参数 | 传统3×105t煤制甲醇 | 绿氢高效耦合新工艺Ⅰ | 绿氢高效耦合新工艺Ⅱ |
|---|---|---|---|
| 甲醇产量/t | 3×105 | 6.549×105 | 6.549×105 |
| 原料煤消耗量/t | 4.269×105 | 4.269×105 | 4.269×105 |
| 直接CO2排放量/t | 6.431×105 | 1.459×105 | 3.770×105 |
| 生产每吨甲醇耗煤量/t·t-1 | 1.42 | 0.65 | 0.65 |
| 单位甲醇碳排放强度/t·t-1 | 5.92 | 2.20 | 2.58 |
| 绿氢消耗量(标准状况)/m³·h-1 | 0 | 105241 | 35431 |
| 碳元素利用率/% | 38.74 | 84.56 | 67.60 |
| 参数 | 传统3×105t煤制甲醇 | 绿氢高效耦合新工艺Ⅰ | 绿氢高效耦合新工艺Ⅱ |
|---|---|---|---|
| 甲醇产量/t | 3×105 | 6.549×105 | 6.549×105 |
| 原料煤消耗量/t | 4.269×105 | 4.269×105 | 4.269×105 |
| 直接CO2排放量/t | 6.431×105 | 1.459×105 | 3.770×105 |
| 生产每吨甲醇耗煤量/t·t-1 | 1.42 | 0.65 | 0.65 |
| 单位甲醇碳排放强度/t·t-1 | 5.92 | 2.20 | 2.58 |
| 绿氢消耗量(标准状况)/m³·h-1 | 0 | 105241 | 35431 |
| 碳元素利用率/% | 38.74 | 84.56 | 67.60 |
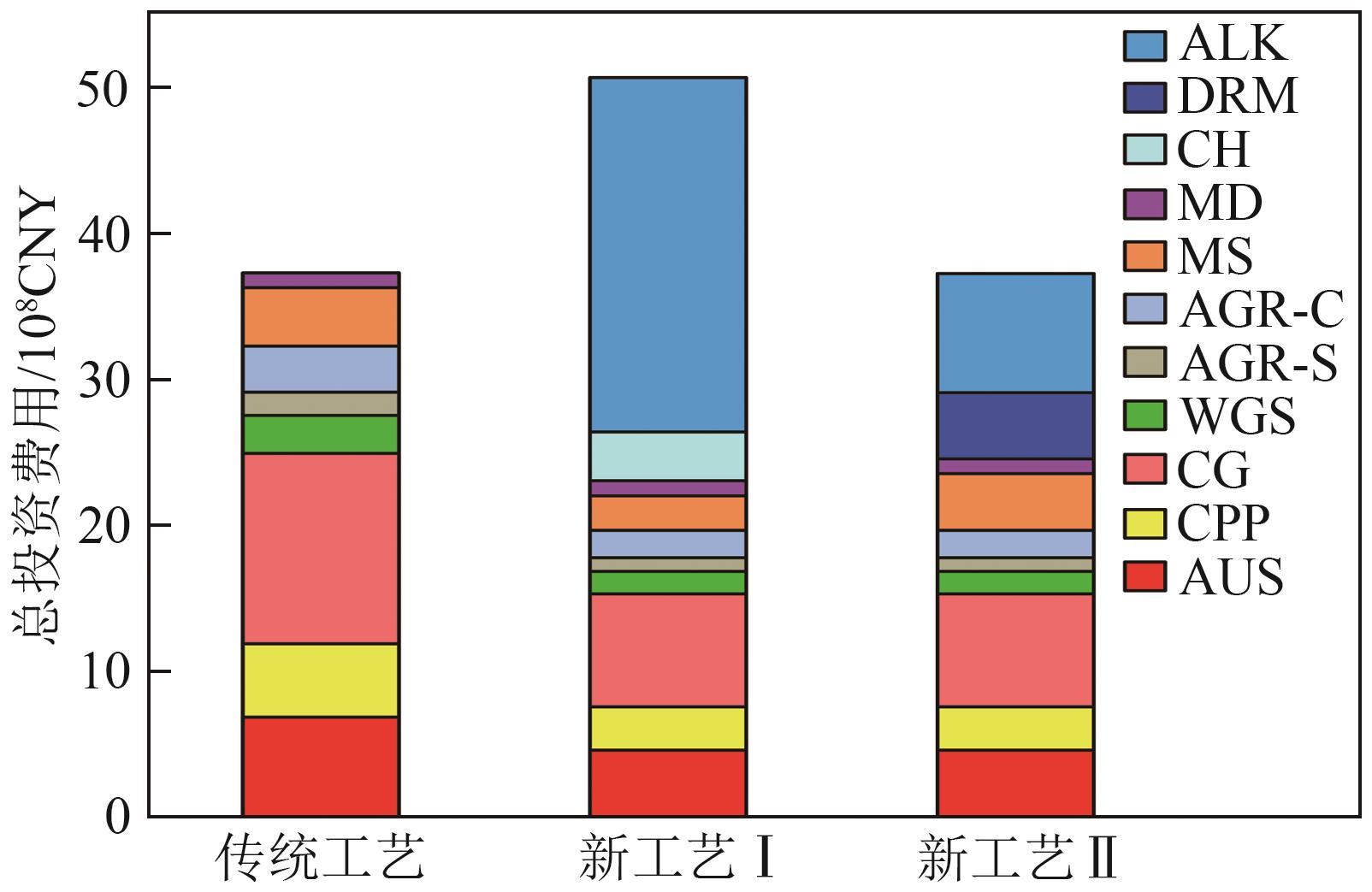
| 投资组成 | 本文基准/% |
|---|---|
| 直接费用 | 67 |
| 设备费用 | 30 |
| 安装费用 | 8 |
| 仪表和控制费用 | 5 |
| 管道费用 | 5 |
| 电器费用 | 6 |
| 厂房及铺设费用 | 12 |
| 土地费用 | 1 |
| 间接费用 | 33 |
| 工程设计和监督 | 11 |
| 施工费和包工费 | 15 |
| 不可预见费用 | 7 |
| 流动资金 | 15 |
| 总投资 | 115 |
| 投资组成 | 本文基准/% |
|---|---|
| 直接费用 | 67 |
| 设备费用 | 30 |
| 安装费用 | 8 |
| 仪表和控制费用 | 5 |
| 管道费用 | 5 |
| 电器费用 | 6 |
| 厂房及铺设费用 | 12 |
| 土地费用 | 1 |
| 间接费用 | 33 |
| 工程设计和监督 | 11 |
| 施工费和包工费 | 15 |
| 不可预见费用 | 7 |
| 流动资金 | 15 |
| 总投资 | 115 |
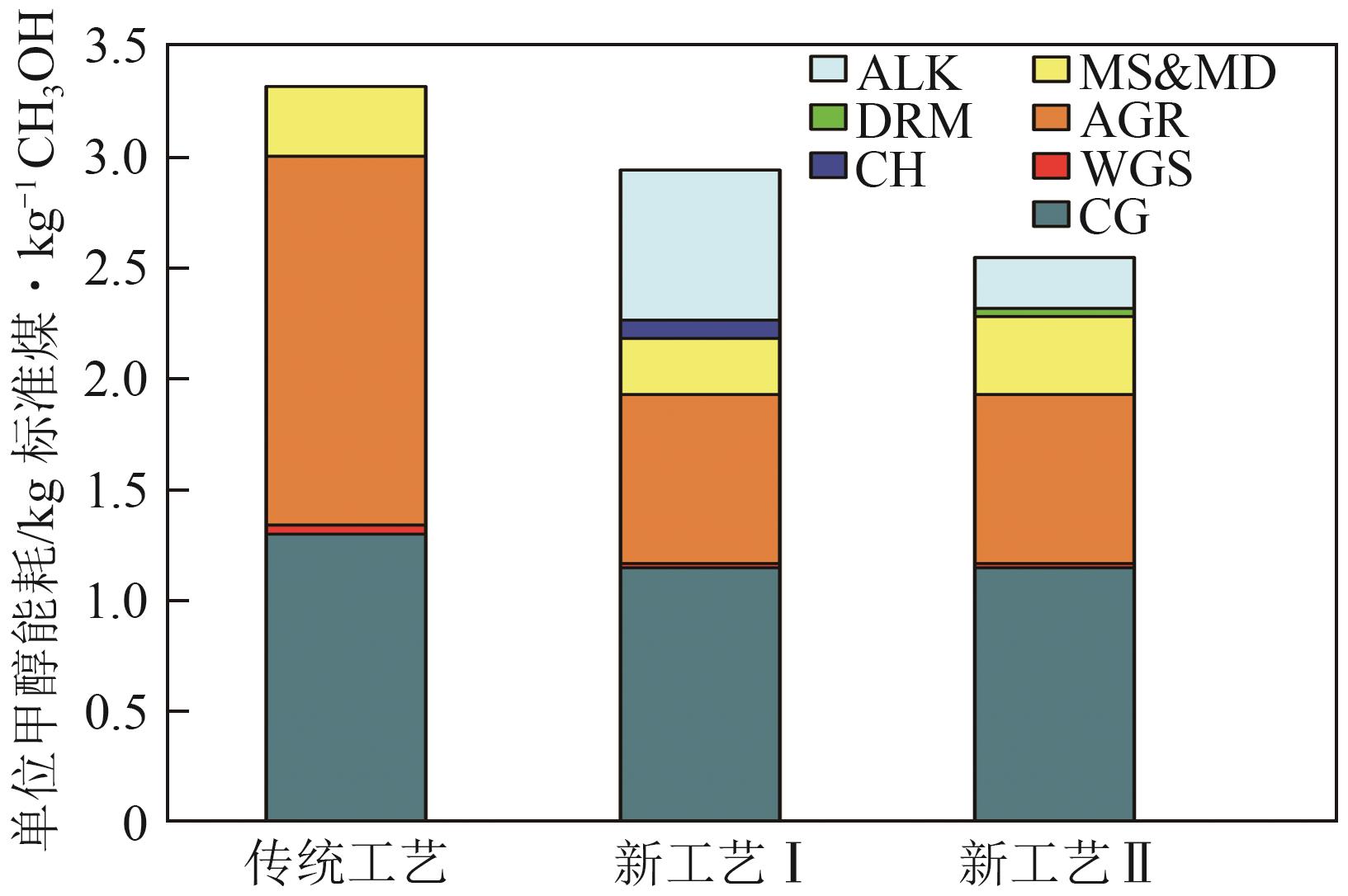
| 单元 | 基准物 | 基准规模 | n | γ | 基准投资/CNY |
|---|---|---|---|---|---|
| 空气分离(AUS) | 供氧量 | 21.3kg/s | 0.50 | 0.50 | 283×106 |
| 煤预处理(CPP) | 日给煤量 | 27.4kg/s | 0.67 | 0.65 | 180×106 |
| 煤气化(CG) | 日给煤量 | 39.2kg/s | 0.67 | 0.80 | 484×106 |
| 水煤气变换(WGS) | 进料热值 | 1450MW | 0.67 | 0.65 | 244×106 |
| 酸性气体净化脱硫(AGR-S) | 硫输出量 | 29.3mol/s | 0.67 | 0.65 | 275×106 |
| 酸性气体净化脱碳(AGR-C) | CO2脱除量 | 2064.4mol/s | 0.67 | 0.65 | 203×106 |
| 甲醇合成(MS) | 进料气量 | 10810mol/s | 0.67 | 0.65 | 142×106 |
| 甲醇精馏(MD) | 甲醇进料量 | 3.66kg/s | 0.67 | 0.65 | 12×106 |
| 二氧化碳加氢(CH) | 进料气量 | 15kg/s | 0.65 | 0.65 | 101×106 |
| 甲烷干重整(DRM) | 进料气量 | 555.6mol/s | 0.67 | 0.65 | 214×106 |
| 单元 | 基准物 | 基准规模 | n | γ | 基准投资/CNY |
|---|---|---|---|---|---|
| 空气分离(AUS) | 供氧量 | 21.3kg/s | 0.50 | 0.50 | 283×106 |
| 煤预处理(CPP) | 日给煤量 | 27.4kg/s | 0.67 | 0.65 | 180×106 |
| 煤气化(CG) | 日给煤量 | 39.2kg/s | 0.67 | 0.80 | 484×106 |
| 水煤气变换(WGS) | 进料热值 | 1450MW | 0.67 | 0.65 | 244×106 |
| 酸性气体净化脱硫(AGR-S) | 硫输出量 | 29.3mol/s | 0.67 | 0.65 | 275×106 |
| 酸性气体净化脱碳(AGR-C) | CO2脱除量 | 2064.4mol/s | 0.67 | 0.65 | 203×106 |
| 甲醇合成(MS) | 进料气量 | 10810mol/s | 0.67 | 0.65 | 142×106 |
| 甲醇精馏(MD) | 甲醇进料量 | 3.66kg/s | 0.67 | 0.65 | 12×106 |
| 二氧化碳加氢(CH) | 进料气量 | 15kg/s | 0.65 | 0.65 | 101×106 |
| 甲烷干重整(DRM) | 进料气量 | 555.6mol/s | 0.67 | 0.65 | 214×106 |
| [1] | 李晔, 冯伟扬. 国内外甲醇行业发展现状分析[J]. 化学工业, 2024, 42(2): 15-21. |
| LI Ye, FENG Weiyang. Development situation analysis of methanol industry[J]. Chemical Industry, 2024, 42(2): 15-21. | |
| [2] | QIN Zhen, ZHAI Guofu, WU Xiaomei, et al. Carbon footprint evaluation of coal-to-methanol chain with the hierarchical attribution management and life cycle assessment[J]. Energy Conversion and Management, 2016, 124: 168-179. |
| [3] | 林海周, 罗志斌, 裴爱国, 等. 二氧化碳与氢合成甲醇技术和产业化进展[J]. 南方能源建设, 2020, 7(2): 14-19. |
| LIN Haizhou, LUO Zhibin, PEI Aiguo, et al. Technology and industrialization progress on methanol synthesis from carbon dioxide and hydrogen[J]. Southern Energy Construction, 2020, 7(2): 14-19. | |
| [4] | 陶加. 大连物化所二氧化碳加氢制甲醇中试[N/OL]. 中国化工报, 2019-07-10(2B)[2024-10-17]. . |
| TAO Jia. Dalian Institute of Chemical Physics achieves CO2 hydrogenation to methanol pilot plant[N/OL]. China Chemical Industry News, 2019-07-10(2B)[2024-10-17]. . | |
| [5] | 全球首套规模化太阳燃料合成示范项目试车成功[J]. 能源化工, 2020, 41(1): 21. |
| The world’s first large-scale liquid solar fuel synthesis demonstration project has been successfully tested in the new area[J]. Energy Chemical Industry, 2020, 41(1): 21. | |
| [6] | 田井清. 甲烷干重整催化剂的设计及其在工业尾气转化中的应用研究[D]. 上海: 华东师范大学, 2020. |
| TIAN Jingqing. Design of methane dry reforming catalyst and its application in industrial tail gas conversion[D]. Shanghai: East China Normal University, 2020. | |
| [7] | 余长春, 李然家, 周红军, 等. CH4/CO2干重整转化制合成气及其应用研究[C]//第二届中国石油石化节能减排技术交流大会论文集. 2015: 137-145. |
| YU Changchun, LI Ranjia, ZHOU Hongjun, et al. Study on dry reforming of CH4 and CO2 to syngas and its application[C]//Proceedings of the 2th China Petroleum and Petrochemical Technology Exchange Conference on Energy Conservation and Emission Reduction. 2015: 137-145. | |
| [8] | YANG Xiao, YANG Zhuwei, LI Linsen, et al. Improving the anti-coking ability in the Ni-M(M = Ce, Zr, Co)@SiO2 yolk-shell catalysts for dry reforming of methane[J]. Fuel, 2024, 368: 131541. |
| [9] | 曾纪龙. 大型煤制甲醇的气化和合成工艺选择[J]. 煤化工, 2005, 33(5): 5-9. |
| ZENG Jilong. Selection of gasification and synthesis processes for large scale coal-to-methanol plant[J]. Coal Chemical Industry, 2005, 33(5): 5-9. | |
| [10] | 陈倩, 李士雨, 李金来, 等. 水煤浆气化过程的计算机模拟[J]. 计算机与应用化学, 2012, 29(12): 1425-1428. |
| CHEN Qian, LI Shiyu, LI Jinlai, et al. Simulation of water-coal slurry gasification process[J]. Computers and Applied Chemistry, 2012, 29(12): 1425-1428. | |
| [11] | KISS Anton A, PRAGT J J, VOS H J, et al. Novel efficient process for methanol synthesis by CO2 hydrogenation[J]. Chemical Engineering Journal, 2016, 284: 260-269. |
| [12] | 刘玮, 万燕鸣, 熊亚林, 等. 碳中和目标下电解水制氢关键技术及价格平准化分析[J]. 电工技术学报, 2022, 37(11): 2888-2896. |
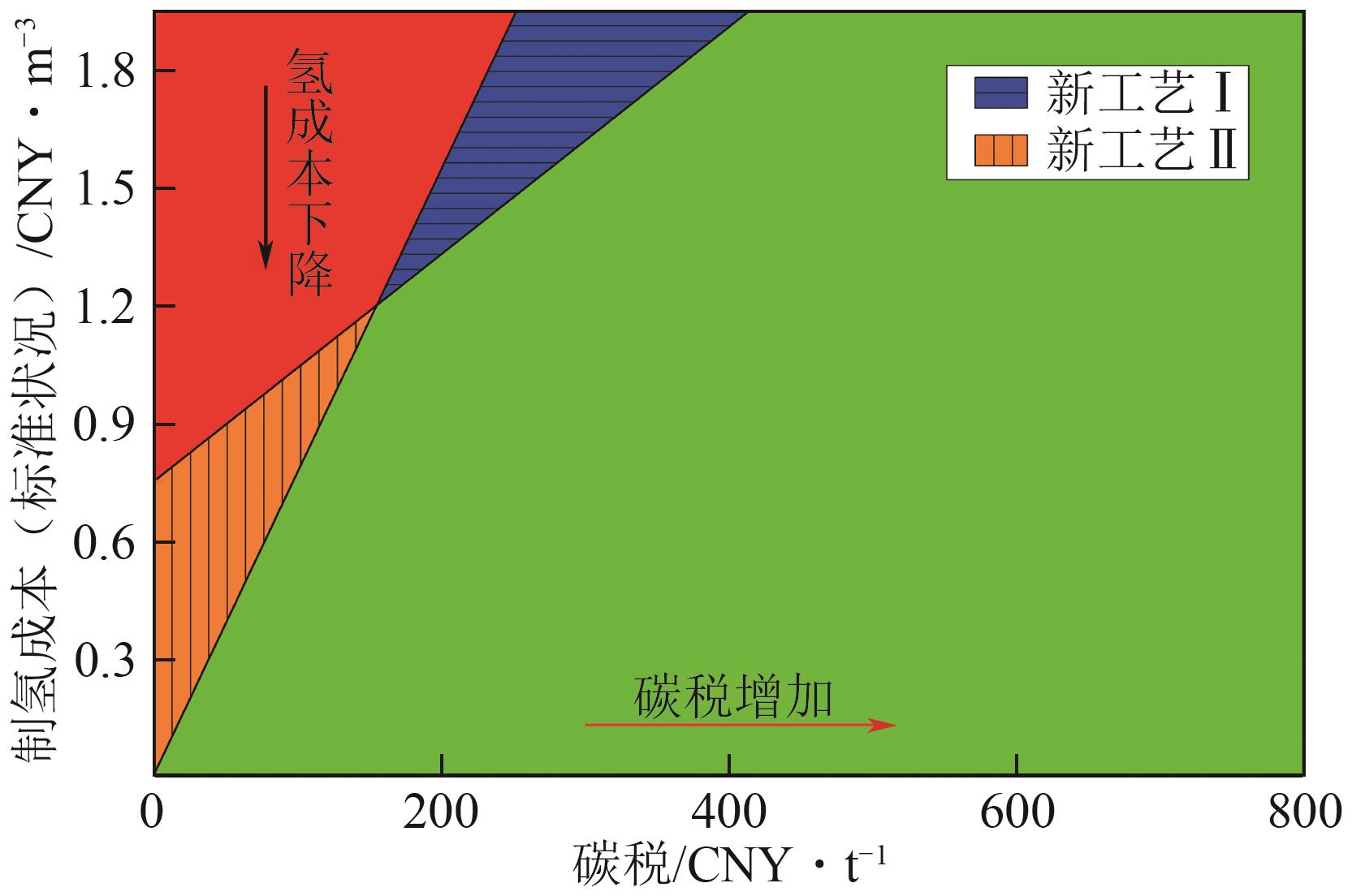
| LIU Wei, WAN Yanming, XIONG Yalin, et al. Key technology of water electrolysis and levelized cost of hydrogen analysis under carbon neutral vision[J]. Transactions of China Electrotechnical Society, 2022, 37(11): 2888-2896. | |
| [13] | FISHER F, TROPSCH H. Conversion of methane into hydrogen and carbon monoxide[J]. Brennst-Chem, 1928, 3(9): 39-46. |
| [14] | BODROV N M, APELBAUM L O, TEMKIN M I. Kinetics of the reactions of methane with steam on the surface of nickel at 400—600℃[J]. Kinetics and Catalysis, 1968, 9(5): 1065-1071. |
| [15] | 钱慧琳, 冉金玲, 何安帮, 等. 二氧化碳-甲烷干气重整反应及其积炭控制的热力学分析[J]. 低碳化学与化工, 2023, 48(5): 55-61. |
| QIAN Huilin, RAN Jinling, HE Anbang, et al. Thermodynamic analysis of carbon dioxide-methane dry reforming and its carbon deposition control[J]. Low-Carbon Chemistry and Chemical Engineering, 2023, 48(5): 55-61. | |
| [16] | 国家市场监督管理总局, 国家标准化管理委员会. 综合能耗计算通则: [S]. 北京: 中国标准出版社, 2020. |
| State Market Regulatory Administration, Standardization Administration of the People’s Republic of China. General rules for calculation of the comprehensive energy consumption: [S]. Beijing: Standards Press of China, 2020. | |
| [17] | ZHANG Jingpeng, LI Zhengwen, ZHANG Zhihe, et al. Techno-economic analysis of integrating a CO2 hydrogenation-to-methanol unit with a coal-to-methanol process for CO2 reduction[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(49): 18062-18070. |
| [18] | 吕伟, 胡雅萍, 曹效军, 等. 煤化工项目碳排放环境影响评价案例分析与研究[J]. 中国科技纵横, 2023, 1(1): 47-52. |
| LU Wei, HU Yaping, CAO Xiaojun, et al. Case study on environmental impact assessment of carbon emission from coal chemical projects[J]. China Science & Technology Overview, 2023, 1(1): 47-52. | |
| [19] | 杨庆, 许思敏, 张大伟, 等. 石油与煤路线制乙二醇过程的技术经济分析[J]. 化工学报, 2020, 71(5): 2164-2172. |
| YANG Qing, XU Simin, ZHANG Dawei, et al. Techno-economic analysis of oil and coal to ethylene glycol processes[J]. CIESC Journal, 2020, 71(5): 2164-2172. | |
| [20] | LI Guang, CHANG Yuxue, LIU Tao, et al. Hydrogen element flow and economic analyses of a coal direct chemical looping hydrogen generation process[J]. Energy, 2020, 206: 118243. |
| [21] | ZHOU Li, HU Shanying, CHEN Dingjiang, et al. Study on systems based on coal and natural gas for producing dimethyl ether[J]. Industrial & Engineering Chemistry Research, 2009, 48(8): 4101-4108. |
| [22] | YANG Siyu, YANG Qingchun, LI Hengchong, et al. An integrated framework for modeling, synthesis, analysis, and optimization of coal gasification-based energy and chemical processes[J]. Industrial & Engineering Chemistry Research, 2012, 51(48): 15763-15777. |
| [23] | 季东, 王健, 王可, 等. 不同CO2捕集技术的CO2耦合绿氢制甲醇工艺研究[J]. 化工学报, 2022, 73(10): 4565-4575. |
| JI Dong, WANG Jian, WANG Ke, et al. Process research of methanol production by CO2 coupled green hydrogen with different CO2 capture technologies[J]. CIESC Journal, 2022, 73(10): 4565-4575. | |
| [24] | 朱超, 田地, 张浩杰, 等. 二氧化碳加氢制甲醇与电解制甲醇系统技术经济性分析[J]. 天然气化工(C1化学与化工), 2023, 48(1): 117-124. |
| ZHU Chao, TIAN Di, ZHANG Haojie, et al. System techno-economic analysis of CO2 hydrogenation to methanol and electrolysis to methanol[J]. Natural Gas Chemical Industry, 2023, 48(1): 117-124. | |
| [25] | REZAEI Ebrahim, DZURYK Stephen. Techno-economic comparison of reverse water gas shift reaction to steam and dry methane reforming reactions for syngas production[J]. Chemical Engineering Research and Design, 2019, 144: 354-369. |
| [26] | 雷昕儒, 孙喆. 煤化学链气化制甲醇工艺模拟与技术经济分析[J]. 天然气化工(C1化学与化工), 2021, 46(1): 99-106. |
| LEI Xinru, SUN Zhe. Process simulation and techno-economic analysis of methanol production via coal chemical looping gasification[J]. Natural Gas Chemical Industry, 2021, 46(1): 99-106. | |
| [27] | 潘楠, 杨晓丽. 国际碳税政策实践发展与经验借鉴[J]. 金融经济, 2024 (1): 77-85. |
| PAN Nan, YANG Xiaoli. Practical development and experience of international carbon tax policy[J]. Finance Economy, 2024 (1): 77-85. |
| [1] | YANG Sen, XUE Zijie, WANG Yufei, ZHAO Liang, XU Chunming. Low carbon transformation and research status of chemical industry based on green hydrogen [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3288-3304. |
| [2] | SUN Zhongshun, LIU Gen, CHENG Chunyu, LI Meixin, YANG Xiantan, WU Zhiqiang, YANG Bolun. Research progress on thermochemical conversion of biomass to green hydrogen [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2667-2682. |
| [3] | GAO Jiangang, JIANG Yapeng, BAO Baoqing, WANG Shuqi, CUI Shuming. Green methanol and green ammonia synthesis by green hydrogen [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 1987-1997. |
| [4] | HUANG Sheng, YANG Zhenli, LI Zhenyu. Analysis of optimization path of developing China's hydrogen industry [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 882-893. |
| [5] | XIANG Hongwei, YANG Yong, LI Yongwang. Transformation and development of coal chemical industry under the goal of carbon neutralization [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1399-1408. |
| [6] | WANG Jijie, HAN Zhe, CHEN Siyu, TANG Chizhou, SHA Feng, TANG Shan, YAO Tingting, LI Can. Liquid sunshine methanol [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1309-1317. |
| [7] | ZHOU Ying, LI Yeqing, ZHOU Hongjun, XU Chunming. Exploration of bio-energy in promoting rural revitalization in China [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6195-6199. |
| [8] | ZHANG Yue,LI Jing,YAN Shenghu,LIU Jianwu,SHEN Jiefa. Effect of Ce loading in CuO-ZnO-Al2O3/HZSM-5 on catalytic performance of dimethyl ether synthesis from carbon dioxide [J]. Chemical Industry and Engineering Progree, 2011, 30(3): 542-. |
| [9] |
ZHANG Luxiang,ZHANG Yongchun,CHEN Shaoyun.
Recent advances and characteristics of methanol and dimethyl ether synthesis from carbon dioxide hydrogenation [J]. Chemical Industry and Engineering Progree, 2010, 29(6): 1041-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||