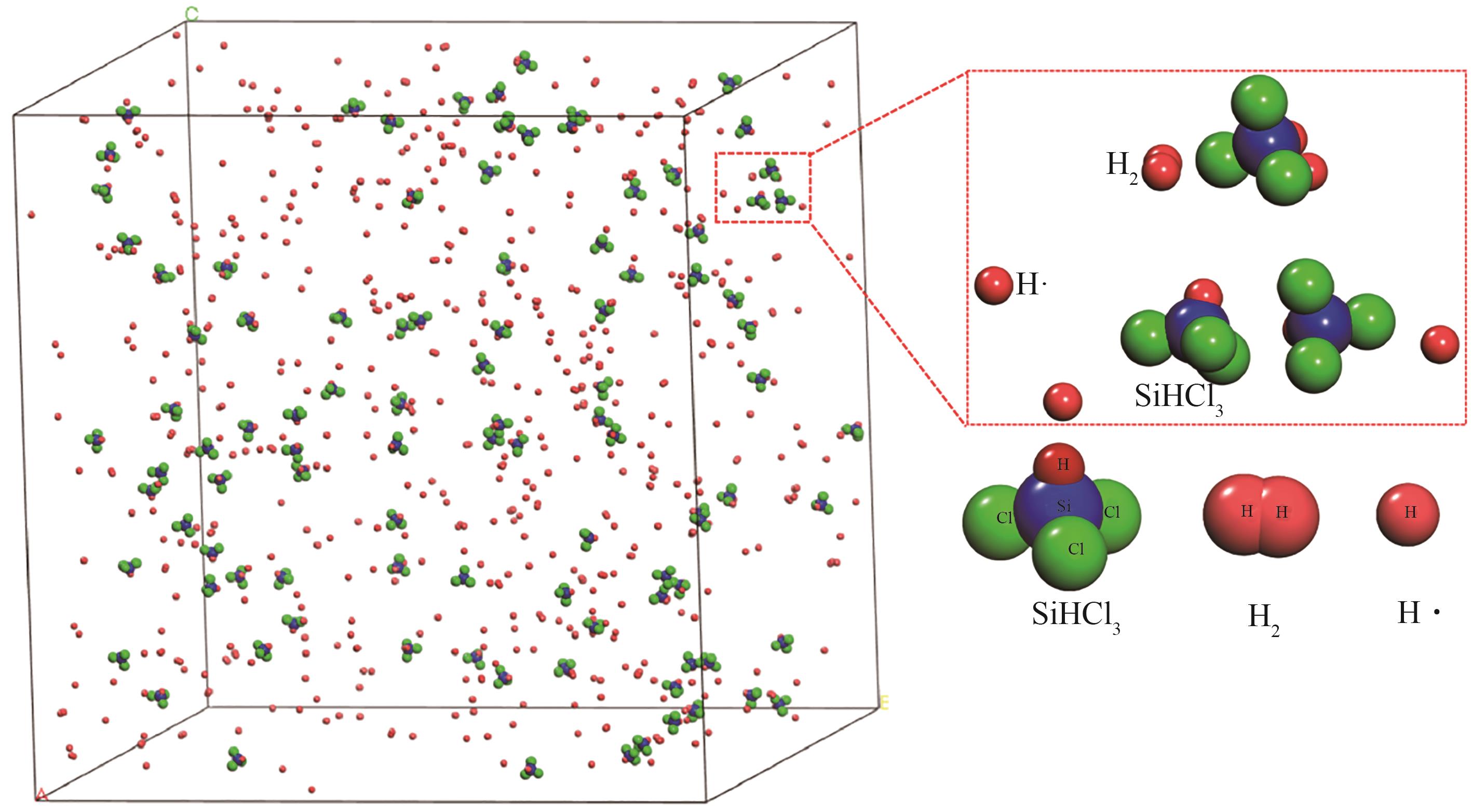
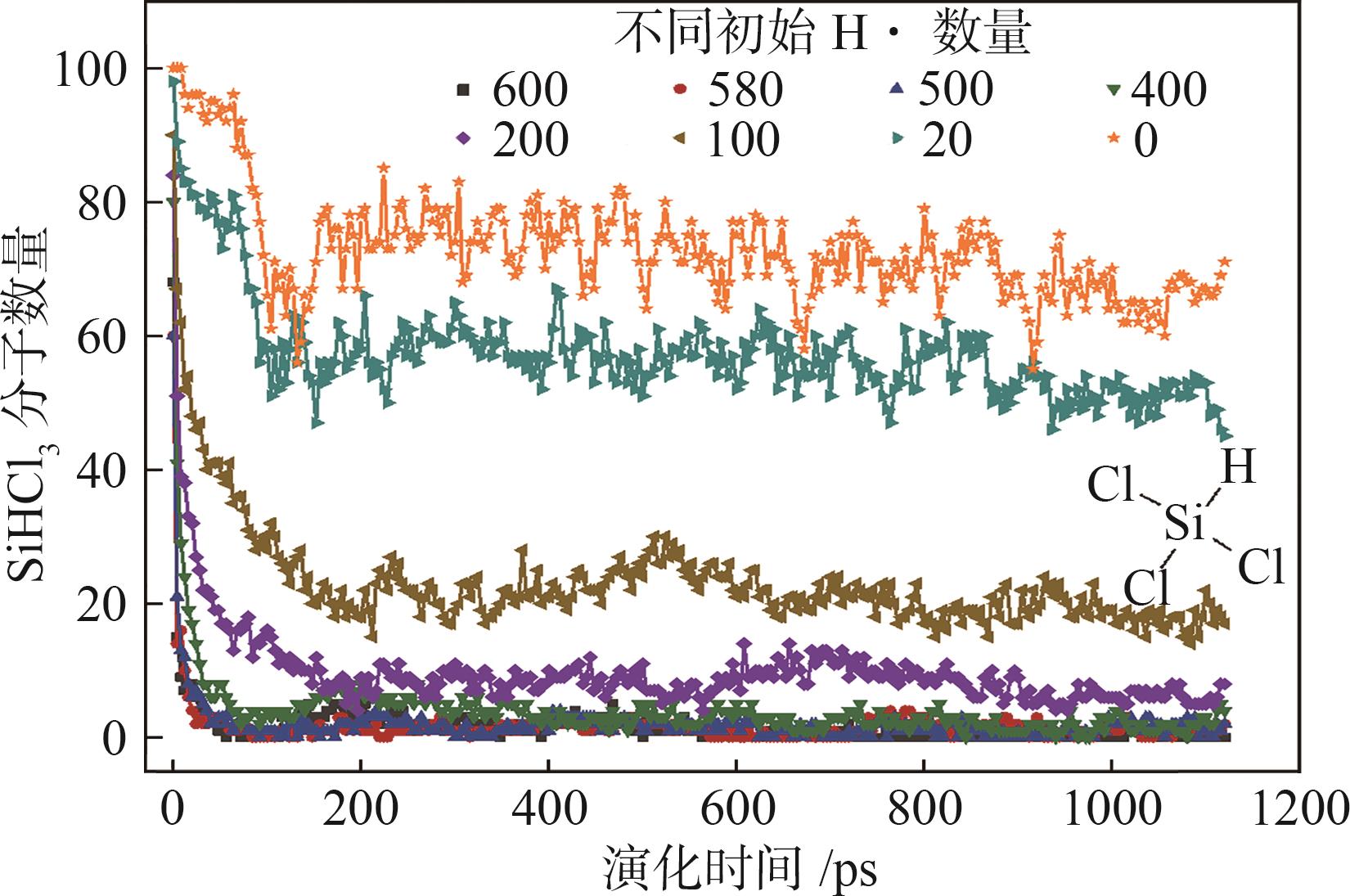
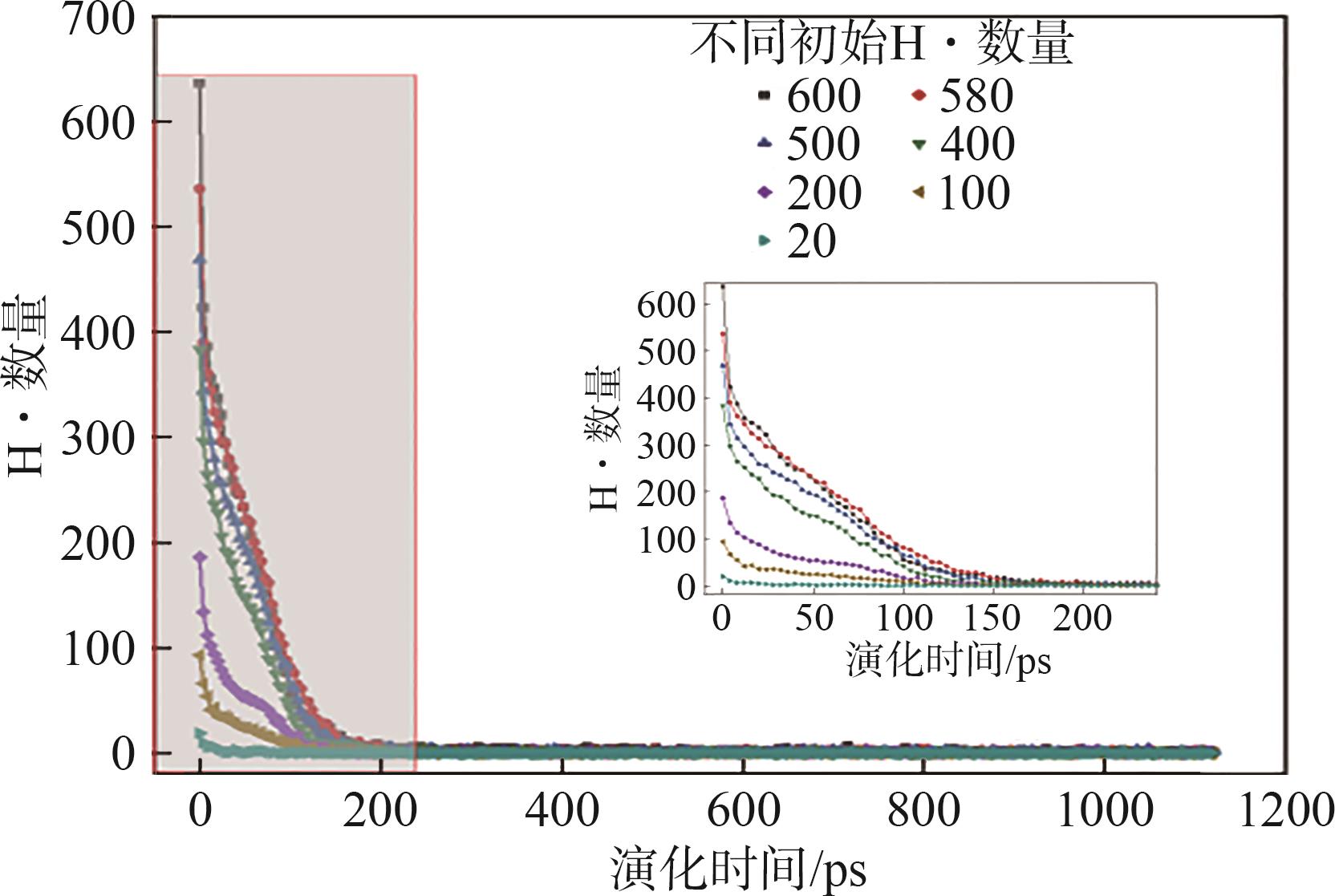
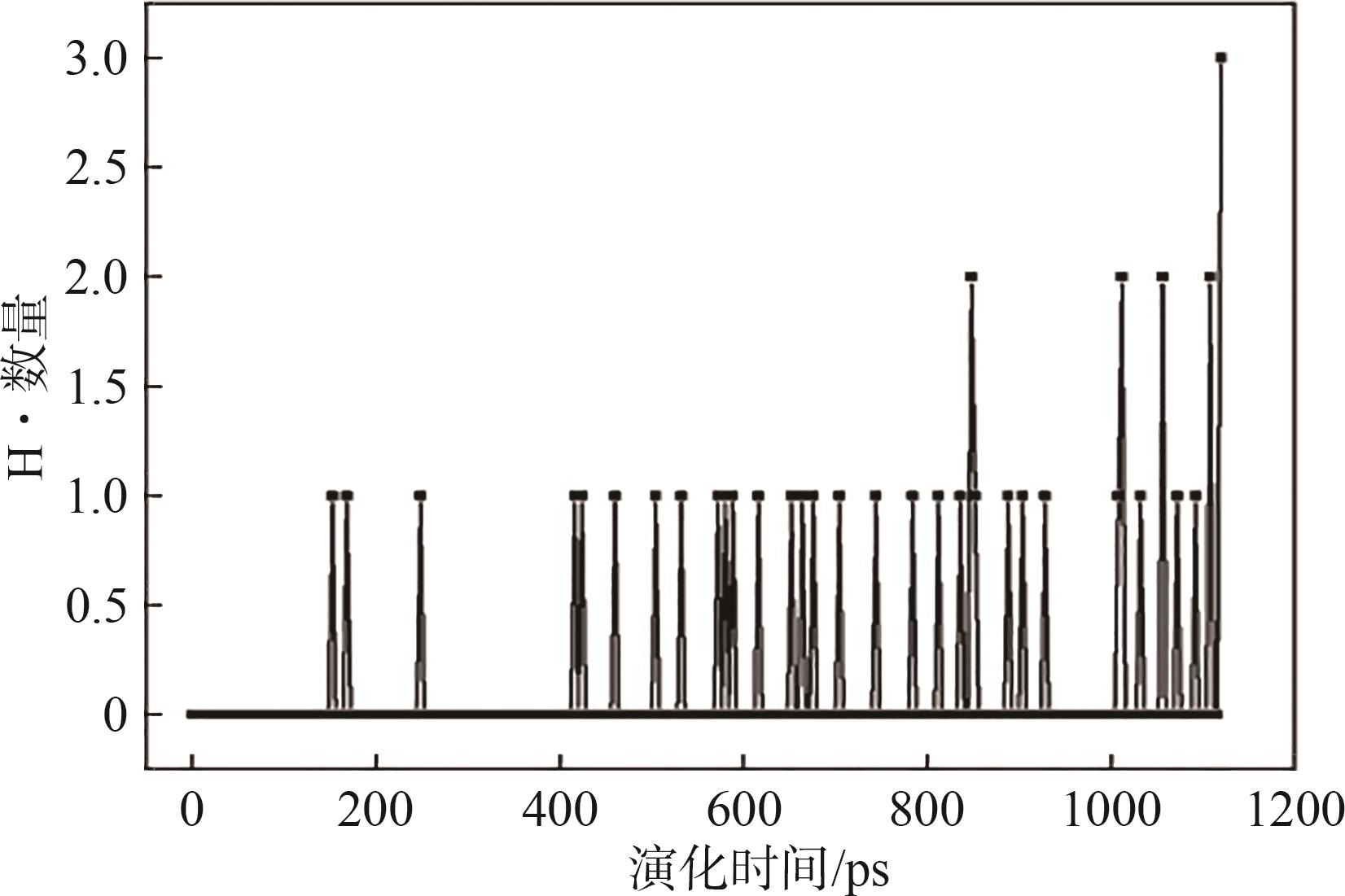
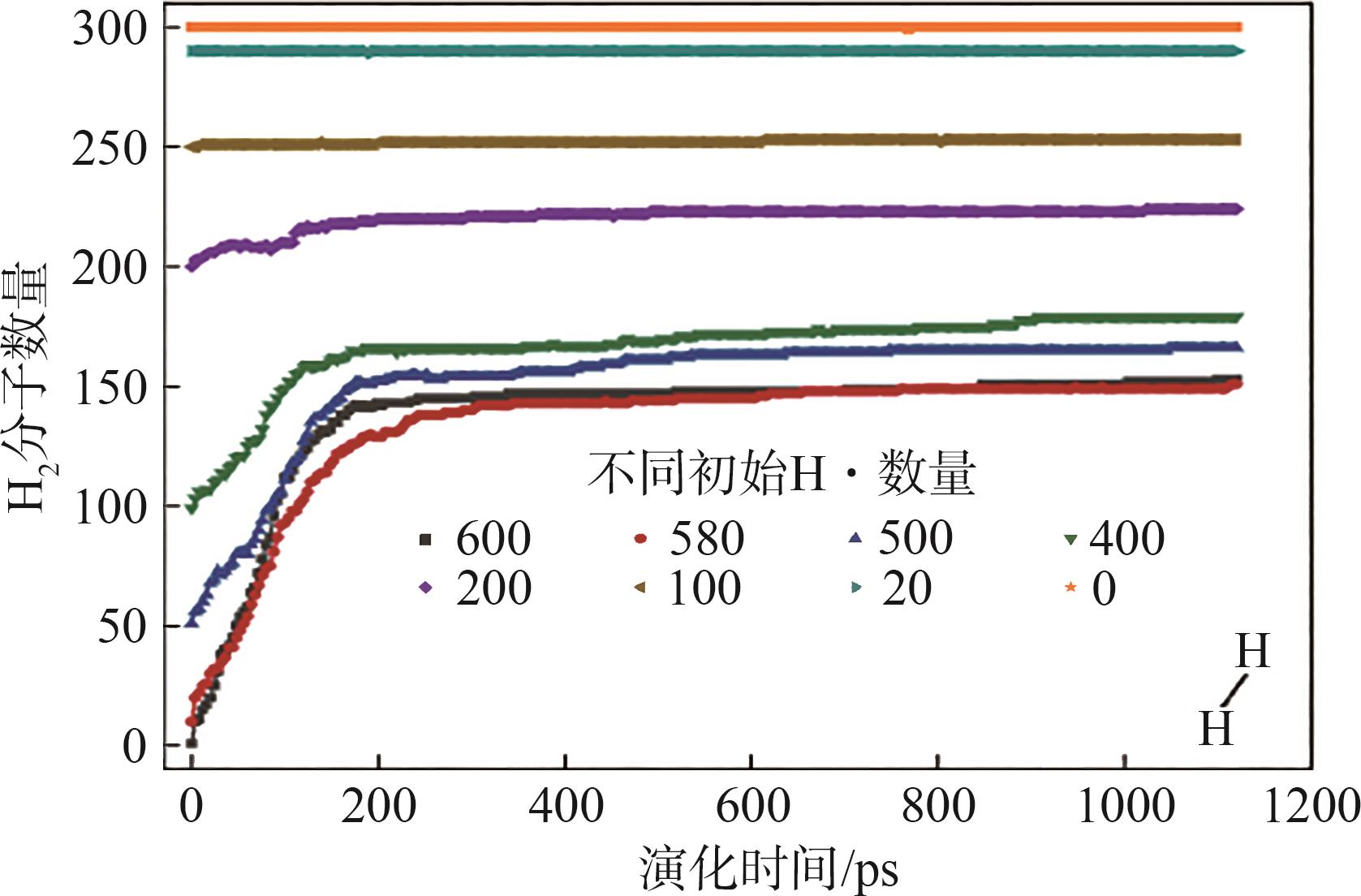
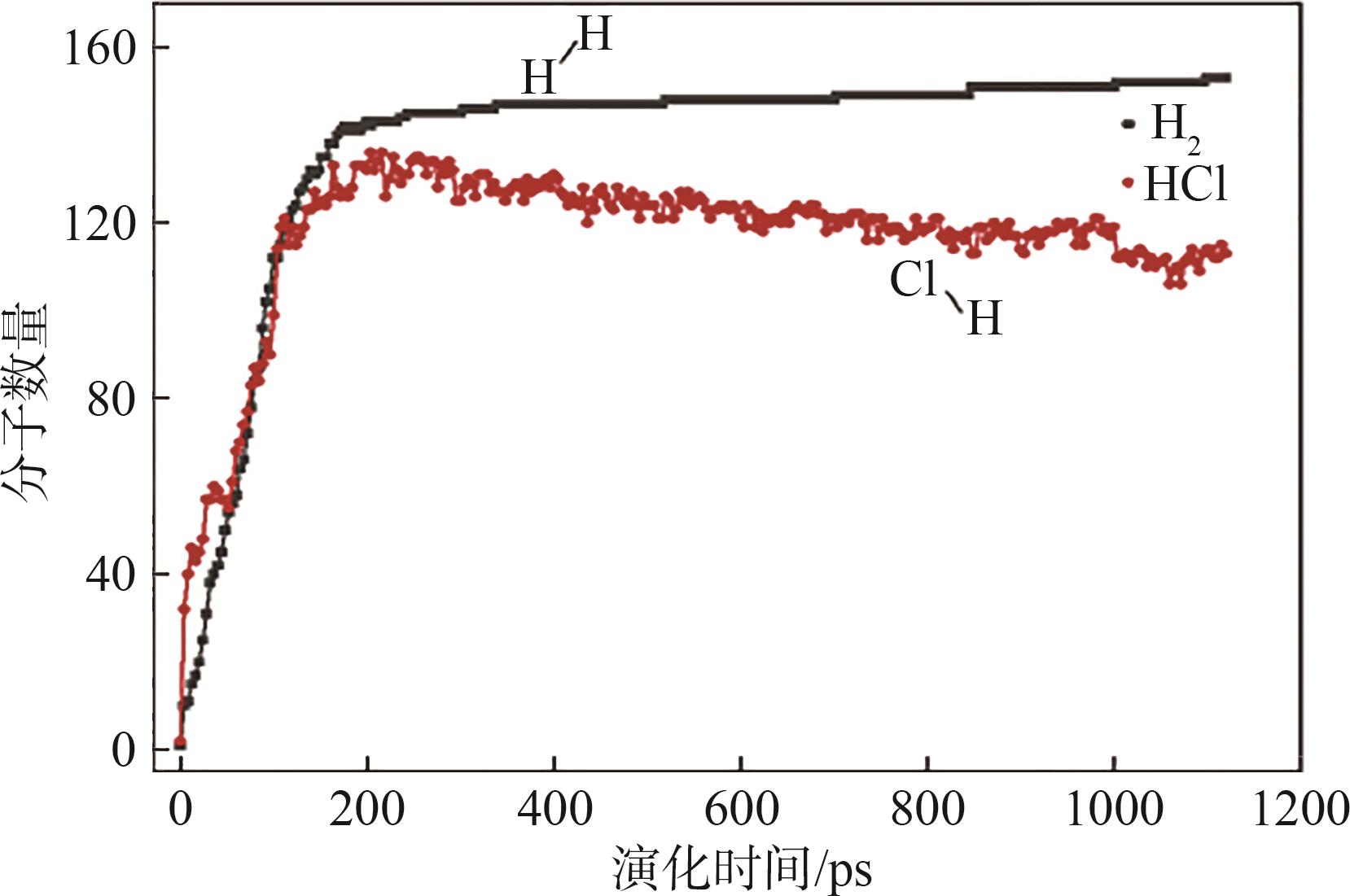
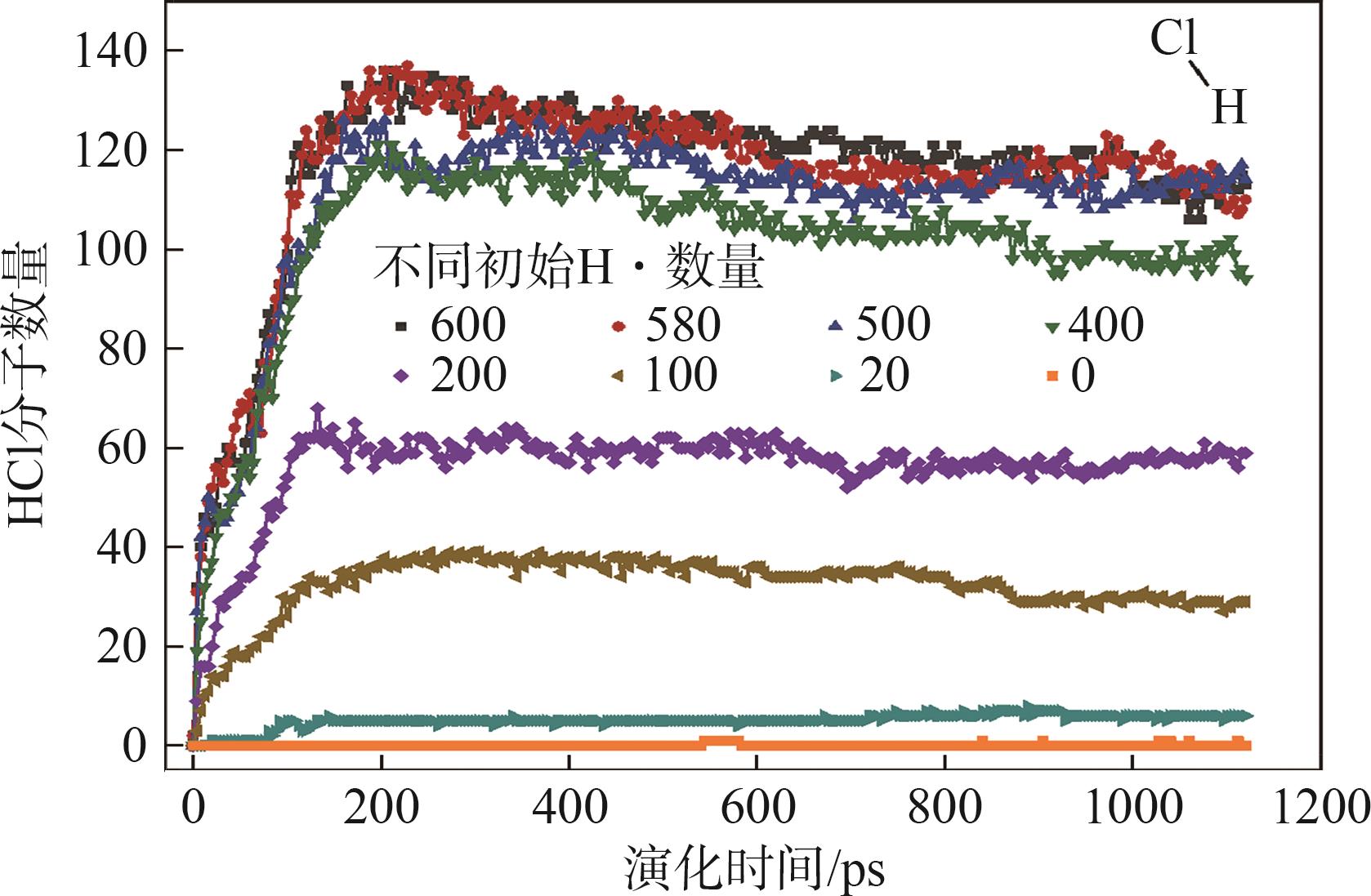
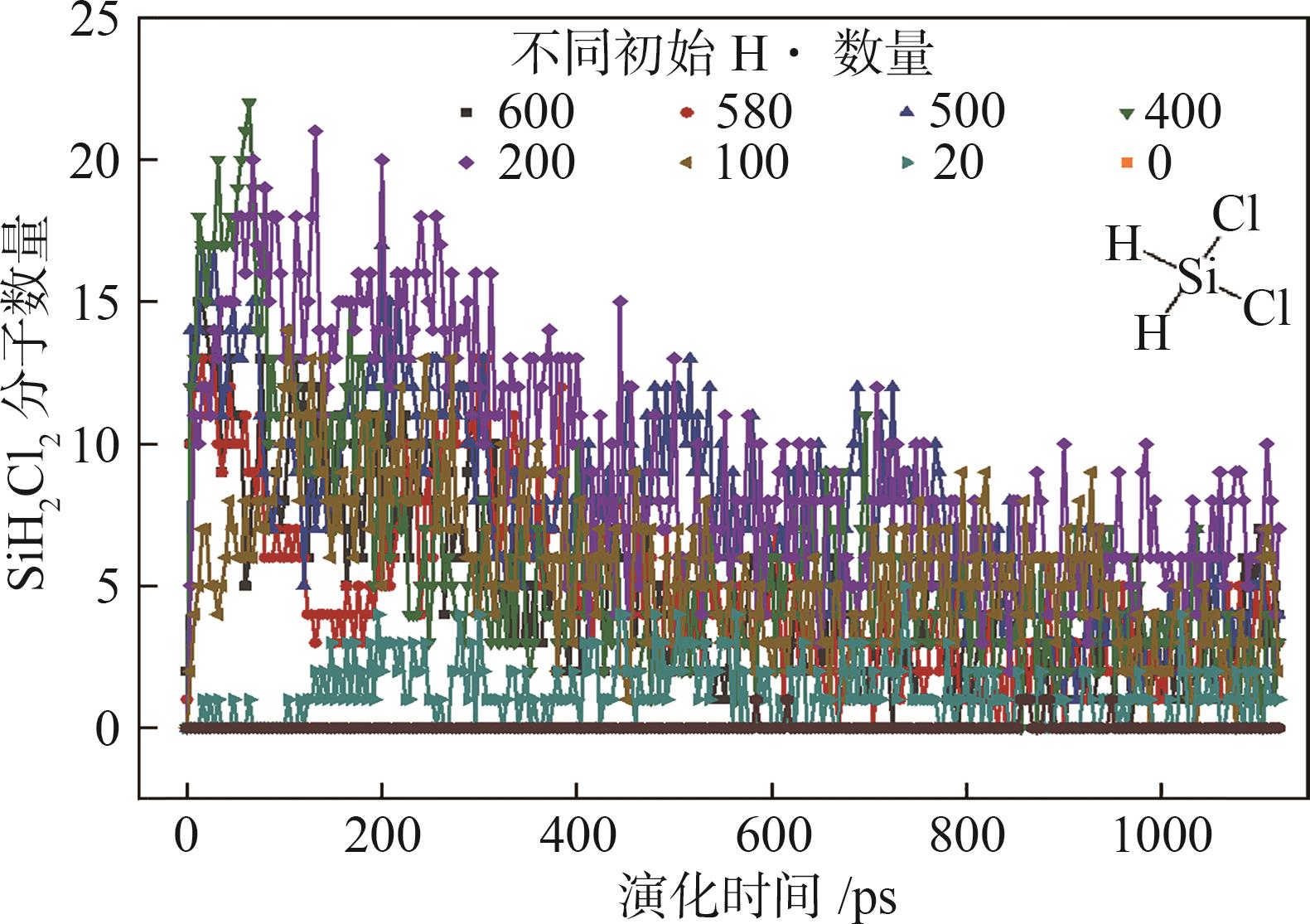
| [1] |
王恩俊, 武锦涛, 银建中, 等. 太阳能级多晶硅生产工艺的现状与发展[J]. 当代化工, 2012, 41(12): 1340-1343, 1347.
|
|
WANG Enjun, WU Jintao, YIN Jianzhong, et al. Current situation and development of solar grade polysilicon production technology[J]. Contemporary Chemical Industry, 2012, 41(12): 1340-1343, 1347.
|
| [2] |
梁骏吾. 光伏产业面临多晶硅瓶颈及对策[J]. 科技导报, 2006, 24(6): 5-7.
|
|
LIANG Junwu. Polycrystalline silicon bottleneck confronting photovoltaic industry and the countermeasures[J]. Science & Technology Review, 2006, 24(6): 5-7.
|
| [3] |
黄友光, 郑静, 潘金花, 等. 四氯化硅热氢化制备三氯氢硅[J]. 现代化工, 2015, 35(10): 75-77, 79.
|
|
HUANG Youguang, ZHENG Jing, PAN Jinhua, et al. Hydrogenation of silicon tetrachloride to trichlorosilane[J]. Modern Chemical Industry, 2015, 35(10): 75-77, 79.
|
| [4] |
倪昊尹, 陈彩霞. 多晶硅化学气相沉积过程气相与表面反应模拟验证[J]. 人工晶体学报, 2015, 44(11): 3083-3089.
|
|
NI Haoyin, CHEN Caixia. Numerical validation of gas and surface reactions for the polysilicon chemical vapor deposition process[J]. Journal of Synthetic Crystals, 2015, 44(11): 3083-3089.
|
| [5] |
黄琪琪, 唐忠利, 黄国强. 三氯氢硅流化床法制备粒状多晶硅的模拟[J]. 化学工业与工程, 2025, 42(4): 161-171.
|
|
HUANG Qiqi, TANG Zhongli, HUANG Guoqiang. Simulation on preparation of preparing granular polysilicon by fluidized-bed trichlorosilane[J]. Chemical Industry and Engineering, 2025, 42(4): 161-171.
|
| [6] |
陈晗. 多晶硅还原炉中的CVD反应体系建模与数值模拟研究[D]. 北京: 北京化工大学, 2023.
|
|
CHEN Han. Modeling and numerical simulation of CVD reaction system in polysilicon reduction furnace[D]. Beijing: Beijing University of Chemical Technology, 2023.
|
| [7] |
MORTIER Wilfried J, GHOSH Swapan K, SHANKAR S. Electronegativity-equalization method for the calculation of atomic charges in molecules[J]. Journal of the American Chemical Society, 1986, 108(15): 4315-4320.
|
| [8] |
FRIESNER Richard A. Ab initio quantum chemistry: Methodology and applications[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(19): 6648-6653.
|
| [9] |
吴胜, 伍刚, 王鹏飞. 芘氧化过程的反应分子动力学模拟[J]. 内燃机学报, 2024, 42(6): 557-562.
|
|
WU Sheng, WU Gang, WANG Pengfei. A study of oxidation process of pyrene by ReaxFF molecular dynamics simulation[J]. Transactions of CSICE, 2024, 42(6): 557-562.
|
| [10] |
陈松岳, 吕鹏. 氧化石墨烯/聚酰亚胺相互作用及反应路径的分子动力学模拟[J]. 化学研究, 2024, 35(4): 311-320.
|
|
CHEN Songyue, Peng LYU. Molecular dynamics simulation of interaction and reaction pathways between graphene oxide and polyimide[J]. Chemical Research, 2024, 35(4): 311-320.
|
| [11] |
贾进章, 田昊, 贾鹏, 等. 内蒙古褐煤的大分子结构模型优化与高温燃烧机理的分子动力学模拟研究[J]. 煤炭转化, 2025, 48(2):35-46.
|
|
JIA Jinzhang, TIAN Hao, JIA Peng, et al. Molecular dynamics simulation study on the optimisation of macromolecular structure model and high-temperature combustion mechanism of Inner Mongolia lignite coal[J]. Coal Conversion, 2025, 48(2): 35-46.
|
| [12] |
LI Yanping, YAN Dazhou, YANG Tao, et al. Revealing the chemical reaction properties of a SiHCl3 pyrolysis system by the ReaxFF molecular dynamics method[J]. ACS Omega, 2022, 7(5): 3900-3916.
|
| [13] |
LIU Song, VAN DUIN Adri C T, VAN DUIN Diana M, et al. Atomistic insights into nucleation and formation of hexagonal boron nitride on nickel from first-principles-based reactive molecular dynamics simulations[J]. ACS Nano, 2017, 11(4): 3585-3596.
|
| [14] |
HONG Sungwook, SHENG Chunyang, KRISHNAMOORTHY Aravind, et al. Chemical vapor deposition synthesis of MoS2 layers from the direct sulfidation of MoO3 surfaces using reactive molecular dynamics simulations[J]. The Journal of Physical Chemistry C, 2018, 122(13): 7494-7503.
|
| [15] |
UENE Naoya, MABUCHI Takuya, ZAITSU Masaru, et al. Reactive force-field molecular dynamics simulation for the surface reaction of SiH x (x=2-4) species on Si(100)-(2×1): H surfaces in chemical vapor deposition processes[J]. Computational Materials Science, 2022, 204: 111193.
|
| [16] |
WANG Xurui, LI Hongyan, LIU Hongli, et al. Growth and defect formation mechanism of CVD-prepared SiC coatings based on cross-scale simulation[J]. Chemical Engineering Journal, 2024, 479: 147652.
|
| [17] |
BERENDSEN H J C, POSTMA J P M, VAN GUNSTEREN W F, et al. Molecular dynamics with coupling to an external bath[J]. The Journal of Chemical Physics, 1984, 81(8): 3684-3690.
|
| [18] |
LIU Jian, LI Xiaoxia, GUO Li, et al. Reaction analysis and visualization of ReaxFF molecular dynamics simulations[J]. Journal of Molecular Graphics and Modelling, 2014, 53: 13-22.
|
 ), YANG Tao1,2,3, WANG Hongxun1,2, ZHANG Cheng1,2, WEN Guosheng1,2,3, HAN Zhicheng1,2, LAN Gongjia1,2, YAN Dazhou1,2,3(
), YANG Tao1,2,3, WANG Hongxun1,2, ZHANG Cheng1,2, WEN Guosheng1,2,3, HAN Zhicheng1,2, LAN Gongjia1,2, YAN Dazhou1,2,3( )
)
 ), 杨涛1,2,3, 王洪勋1,2, 张城1,2, 温国胜1,2,3, 韩治成1,2, 蓝公家1,2, 严大洲1,2,3(
), 杨涛1,2,3, 王洪勋1,2, 张城1,2, 温国胜1,2,3, 韩治成1,2, 蓝公家1,2, 严大洲1,2,3( )
)