Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (6): 3199-3208.DOI: 10.16085/j.issn.1000-6613.2023-0743
• Materials science and technology • Previous Articles
Preparation of nano-spherical LaAlO3 and its fluoride removal performance under acidic environment
LIU Jingdu1( ), YU Guanlong1,2, LONG Zhiqi3, ZHOU Lu1,2, BAO Purui1, TENG Junyi1, DU Chunyan1,2(
), YU Guanlong1,2, LONG Zhiqi3, ZHOU Lu1,2, BAO Purui1, TENG Junyi1, DU Chunyan1,2( )
)
- 1.College of Water and Environmental Engineering, Changsha University of Science and Technology, Changsha 410114, Hunan, China
2.Hunan Key Laboratory of Dongting Lake Water Environment Management and Ecological Restoration, Hunan Environmental Protection River and Lake Dredging Pollution Control Engineering Technology Center, Changsha 410114, Hunan, China
3.GRINM Resources and Environment Tech. Co. , Ltd. , Beijing 101400, China
-
Received:2023-05-06Revised:2023-09-17Online:2024-07-02Published:2024-06-15 -
Contact:DU Chunyan
纳米球状LaAlO3的制备及其在酸性条件下的除氟性能
刘京都1( ), 余关龙1,2, 龙志奇3, 周璐1,2, 包璞瑞1, 滕骏毅1, 杜春艳1,2(
), 余关龙1,2, 龙志奇3, 周璐1,2, 包璞瑞1, 滕骏毅1, 杜春艳1,2( )
)
- 1.长沙理工大学水利与环境工程学院,湖南 长沙 410114
2.洞庭湖水环境治理与生态修复湖南省重点实验室,湖南省环境保护河湖疏浚污染控制工程技术中心,湖南 长沙 410114
3.有研资源环境技术研究院(北京) 有限公司,北京 101400
-
通讯作者:杜春艳 -
作者简介:刘京都(1999—),男,硕士研究生,研究方向为水处理新材料。E-mail:1582804854@qq.com。 -
基金资助:湖南省教育厅重点项目(21A0188);湖南省环境保护河湖疏浚污染控制工程技术中心开放基金(EPD202104);湖南省自然科学基金(2021JJ30728)
CLC Number:
Cite this article
LIU Jingdu, YU Guanlong, LONG Zhiqi, ZHOU Lu, BAO Purui, TENG Junyi, DU Chunyan. Preparation of nano-spherical LaAlO3 and its fluoride removal performance under acidic environment[J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3199-3208.
刘京都, 余关龙, 龙志奇, 周璐, 包璞瑞, 滕骏毅, 杜春艳. 纳米球状LaAlO3的制备及其在酸性条件下的除氟性能[J]. 化工进展, 2024, 43(6): 3199-3208.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0743
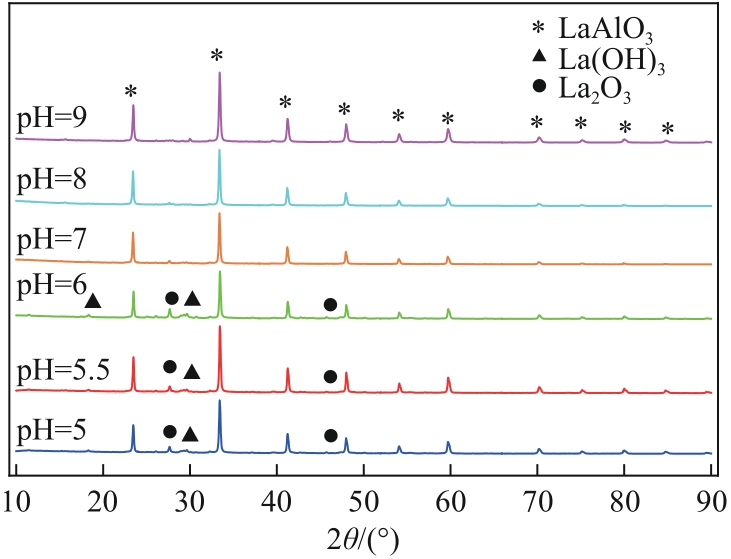
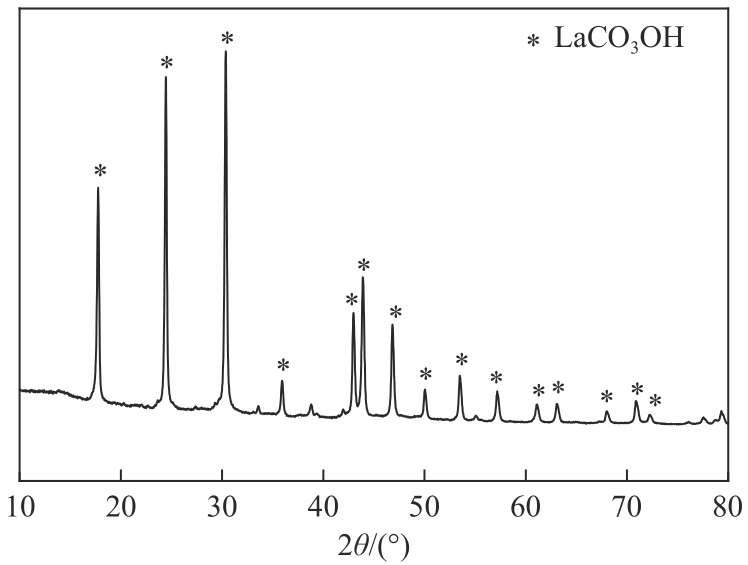
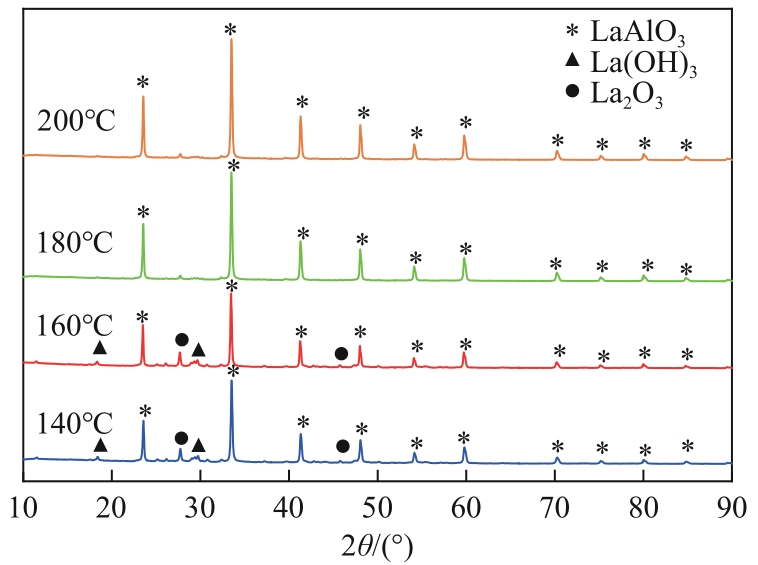
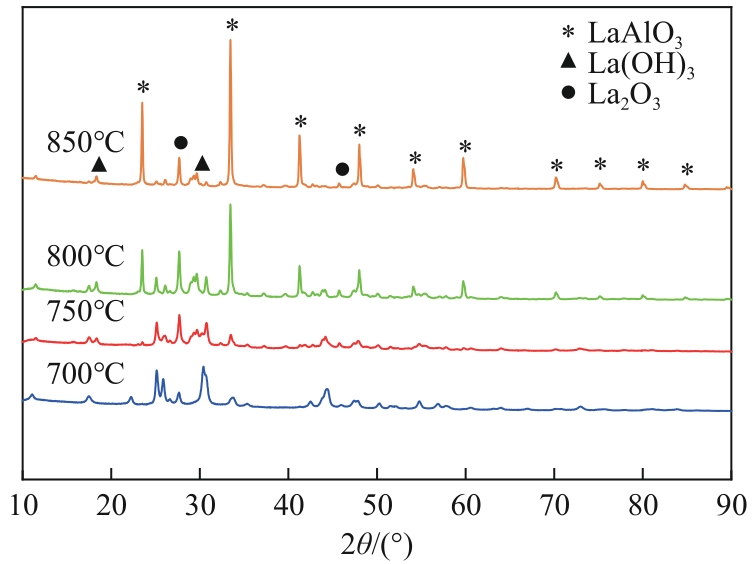
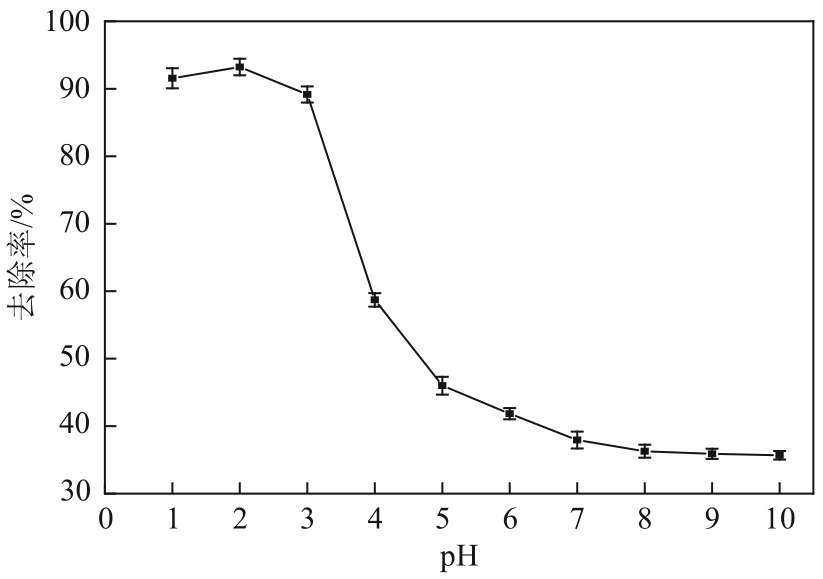
| 沉淀物质 | Ksp | 开始沉淀pH | 完全沉淀pH |
|---|---|---|---|
| Al(OH)3 | 1.3×10-33 | 3.42 | 4.70 |
| La(OH)3 | 2.67×10-30 | 4.52 | 5.81 |
| La2(CO3)3 | 1.1×10-30 | 7.51 | 8.80 |
| LaCO3OH | — | >7.51 | — |
| 沉淀物质 | Ksp | 开始沉淀pH | 完全沉淀pH |
|---|---|---|---|
| Al(OH)3 | 1.3×10-33 | 3.42 | 4.70 |
| La(OH)3 | 2.67×10-30 | 4.52 | 5.81 |
| La2(CO3)3 | 1.1×10-30 | 7.51 | 8.80 |
| LaCO3OH | — | >7.51 | — |
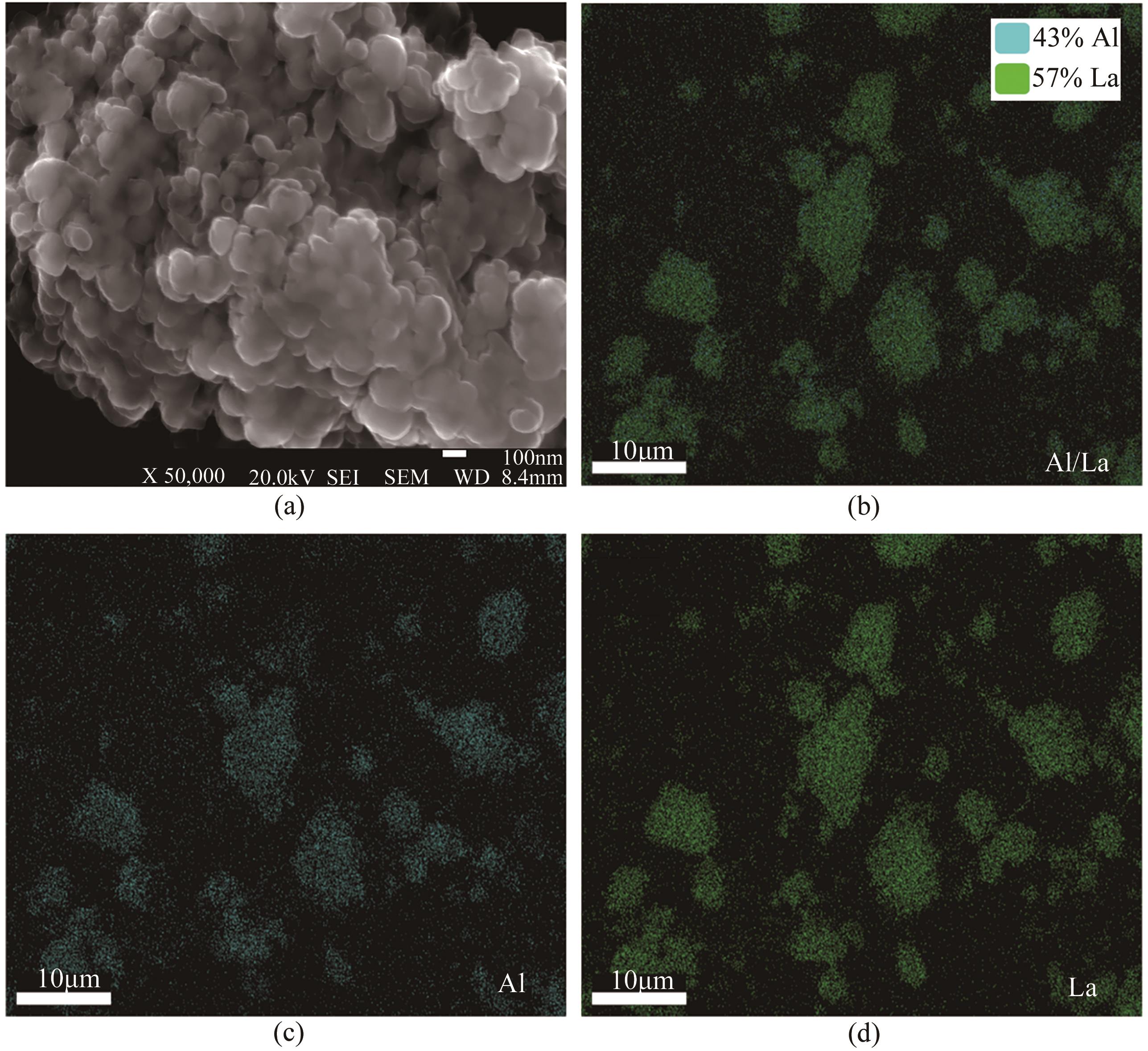
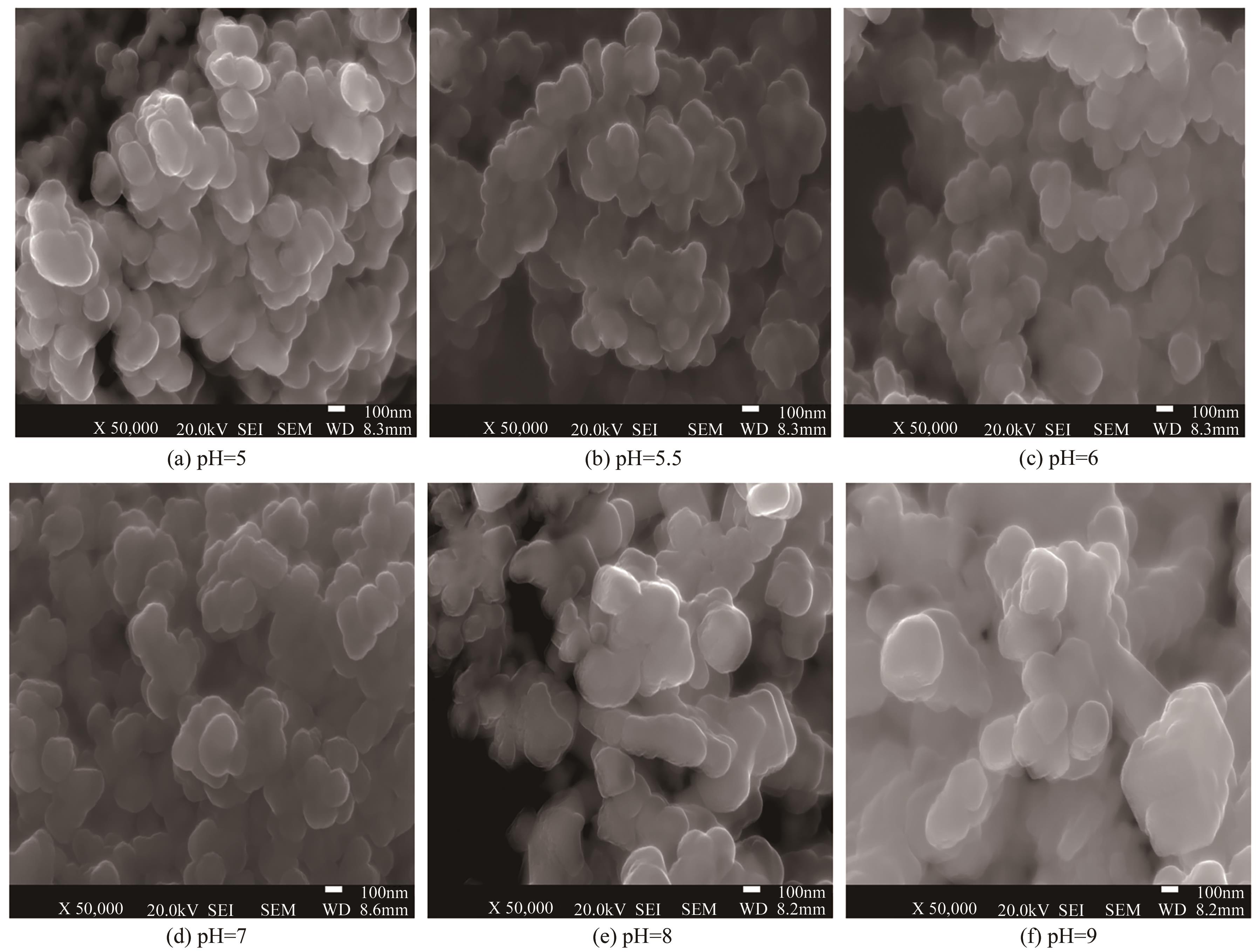
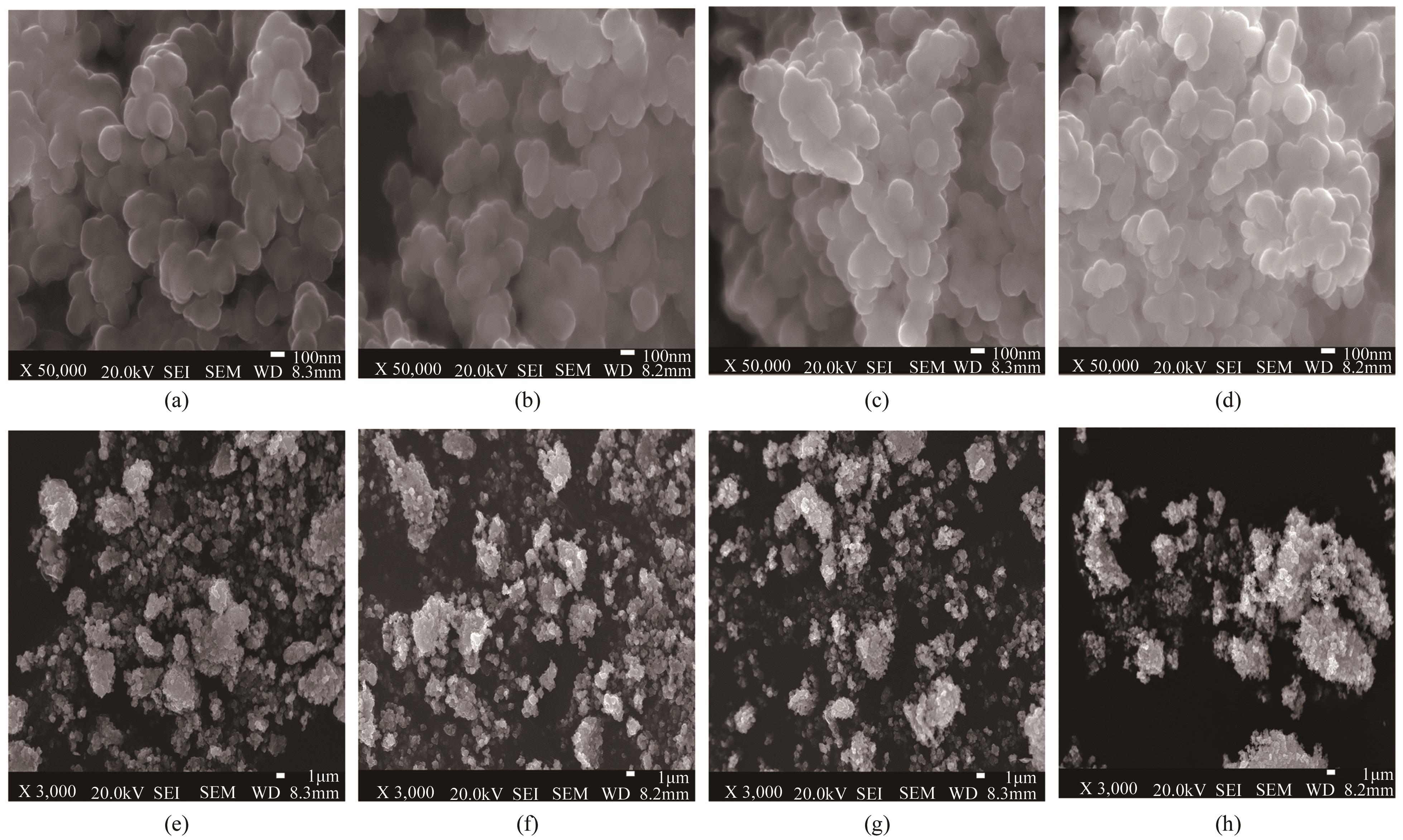
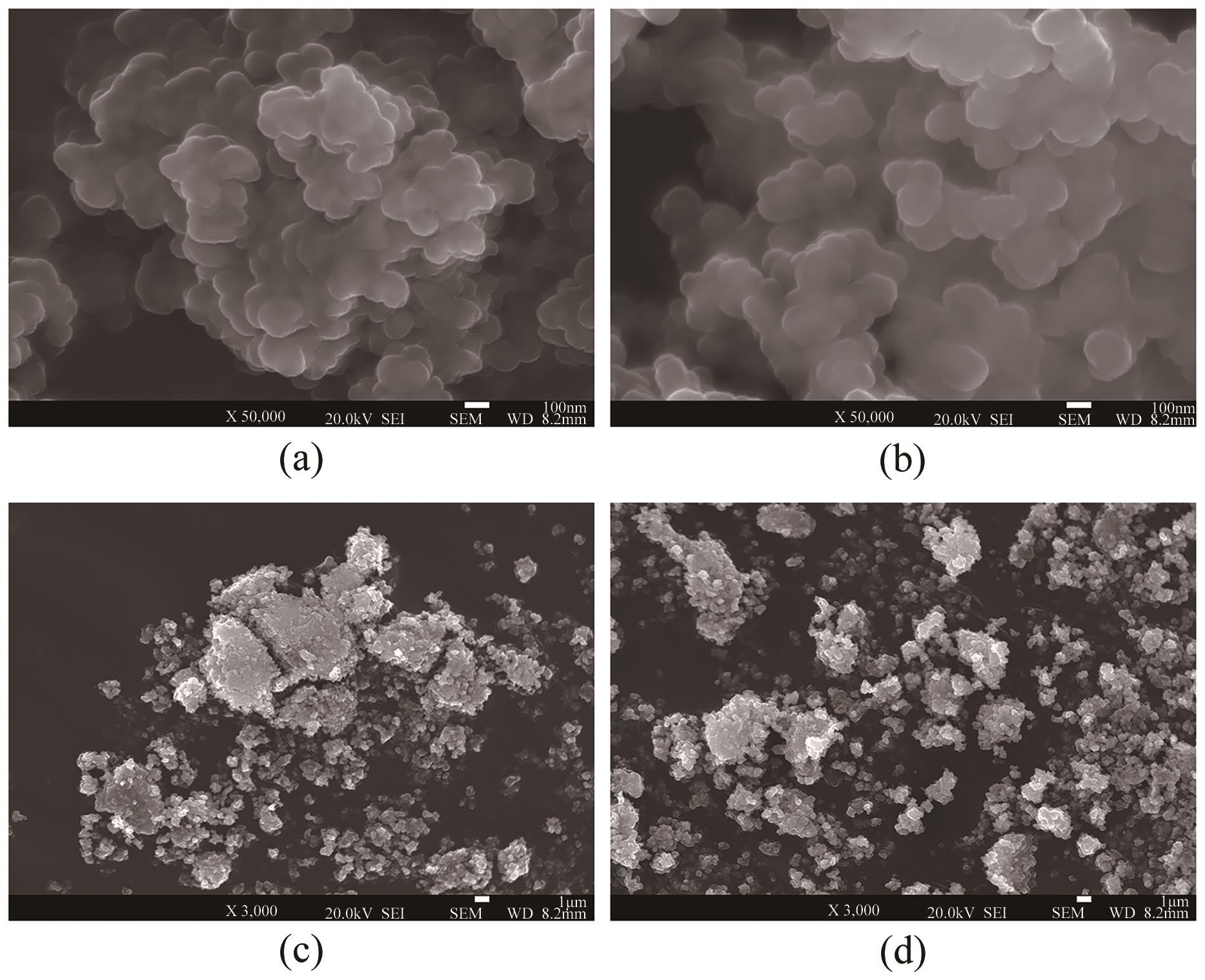
| pH | 晶粒粒径/nm | 比表面积/m2·g-1 | 孔径/nm | 晶粒特征 |
|---|---|---|---|---|
| 5 | 240 | 9.352 | 3.408 | 密集 |
| 6 | 220 | 13.540 | 3.072 | 疏松 |
| 9 | 300 | 3.761 | 3.840 | 结块 |
| pH | 晶粒粒径/nm | 比表面积/m2·g-1 | 孔径/nm | 晶粒特征 |
|---|---|---|---|---|
| 5 | 240 | 9.352 | 3.408 | 密集 |
| 6 | 220 | 13.540 | 3.072 | 疏松 |
| 9 | 300 | 3.761 | 3.840 | 结块 |
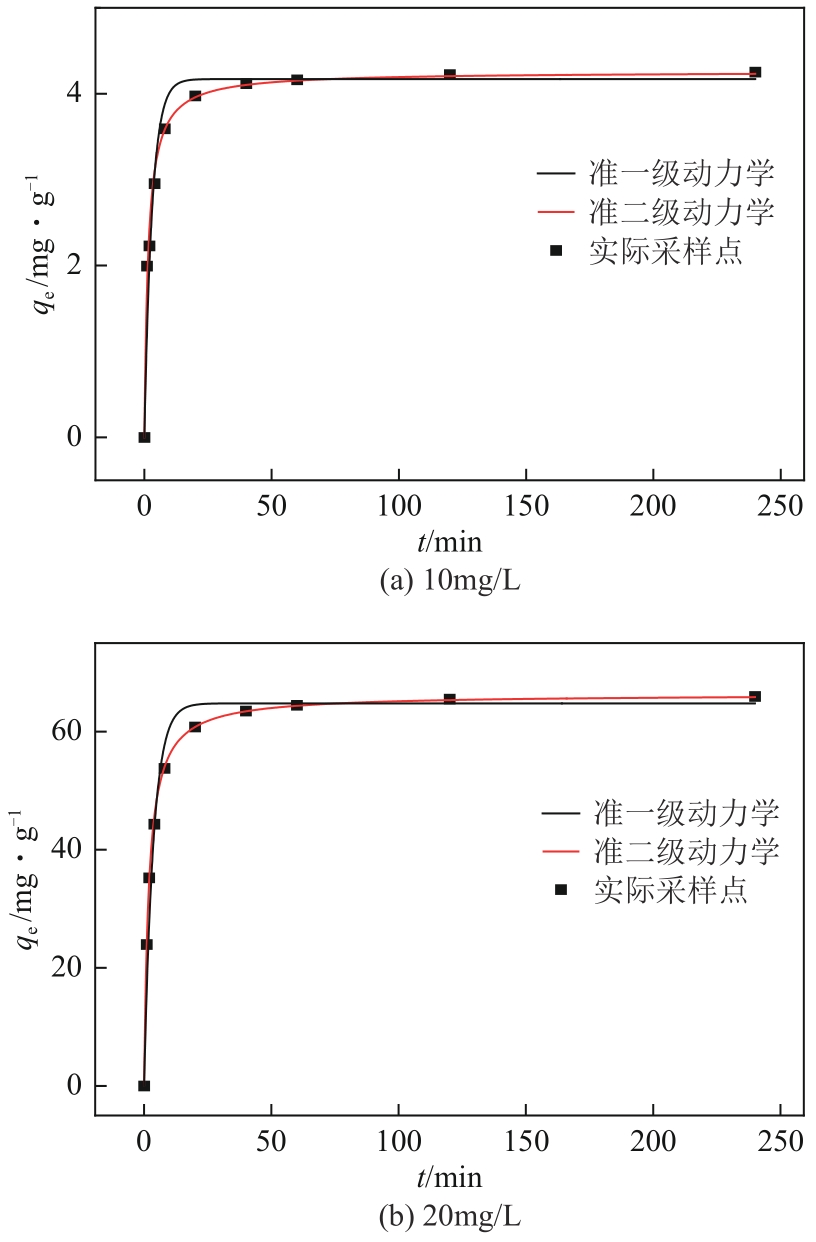
| 准一级动力学模型 | 准二级动力学模型 | Elovich模型 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C0/mg·L-1 | k1/min-1 | R2 | C0/mg·L-1 | K2/g·mg-1·min-1 | qe/mg·g-1 | R2 | C0/mg·L-1 | α/mg·g-1·min-1 | β/g·mg-1 | R2 | ||||||
| 10 | 0.3291 | 0.9108 | 10 | 0.1555 | 4.25 | 0.9904 | 10 | 58871.4042 | 2.17 | 0.8583 | ||||||
| 200 | 0.3615 | 0.9771 | 200 | 0.0082 | 66.34 | 0.9995 | 200 | 119606.6163 | 0.04 | 0.8618 | ||||||
| 内扩散模型 | 外扩散模型 | |||||||||||||||
| C0/mg·L-1 | Kid/mg·g-1·min-0.5 | C | R2 | C0/mg·L-1 | Kp/min-1 | R2 | ||||||||||
| 10 | 0.4614 | 1.9674 | 0.5208 | 10 | 0.0051 | 0.5639 | ||||||||||
| 200 | 9.2286 | 23.0950 | 0.5234 | 200 | 0.0026 | 0.5113 | ||||||||||
| 准一级动力学模型 | 准二级动力学模型 | Elovich模型 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C0/mg·L-1 | k1/min-1 | R2 | C0/mg·L-1 | K2/g·mg-1·min-1 | qe/mg·g-1 | R2 | C0/mg·L-1 | α/mg·g-1·min-1 | β/g·mg-1 | R2 | ||||||
| 10 | 0.3291 | 0.9108 | 10 | 0.1555 | 4.25 | 0.9904 | 10 | 58871.4042 | 2.17 | 0.8583 | ||||||
| 200 | 0.3615 | 0.9771 | 200 | 0.0082 | 66.34 | 0.9995 | 200 | 119606.6163 | 0.04 | 0.8618 | ||||||
| 内扩散模型 | 外扩散模型 | |||||||||||||||
| C0/mg·L-1 | Kid/mg·g-1·min-0.5 | C | R2 | C0/mg·L-1 | Kp/min-1 | R2 | ||||||||||
| 10 | 0.4614 | 1.9674 | 0.5208 | 10 | 0.0051 | 0.5639 | ||||||||||
| 200 | 9.2286 | 23.0950 | 0.5234 | 200 | 0.0026 | 0.5113 | ||||||||||
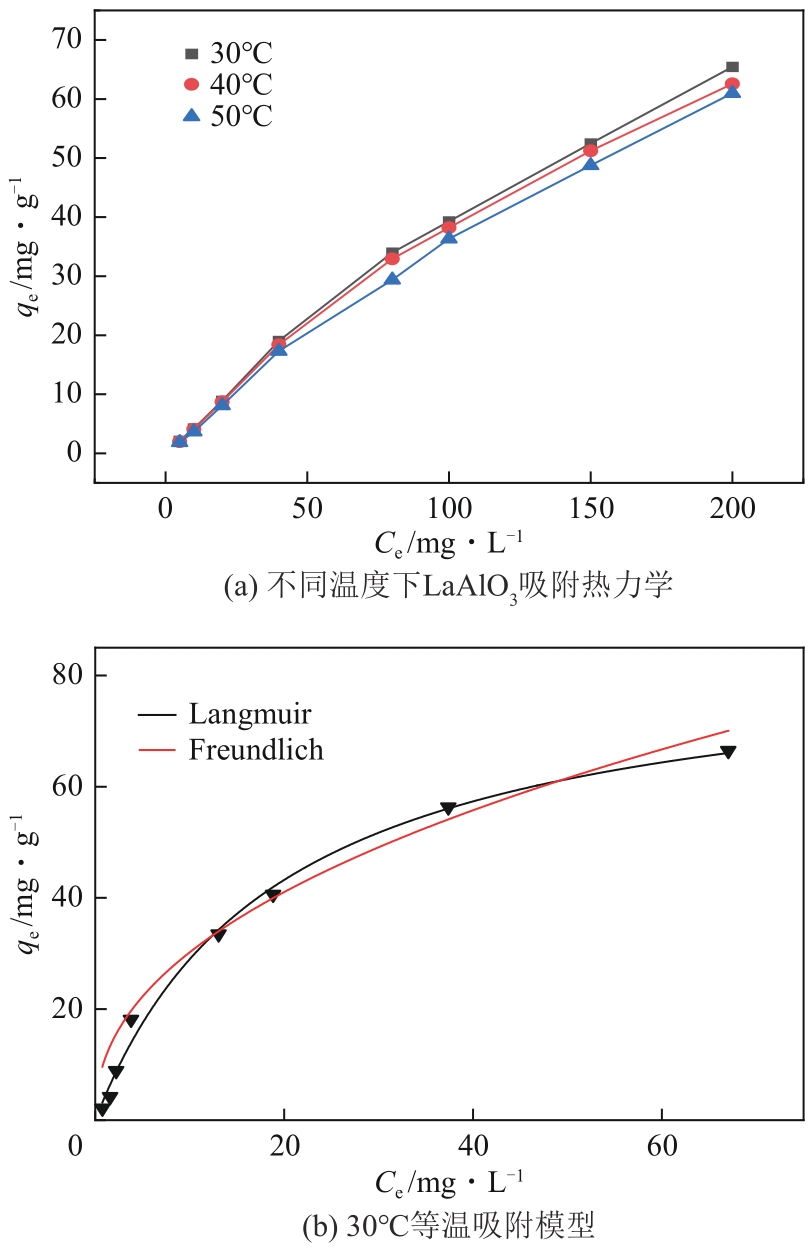
| 温度 /℃ | Langmuir | Freundlich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | qm/mg·g-1 | b/L·mg-1 | R2 | KF/mg1-(1/n)·L1/n ·g-1 | n | R2 | BT/J·mol-1 | KT/L·mg-1 | ||
| 30 | 0.994 | 85.21 | 0.052 | 0.968 | 8.116 | 1.939 | 0.9688 | 14.94 | 0.9734 | |
| 40 | 0.991 | 75.74 | 0.050 | 0.966 | 7.225 | 1.973 | 0.9844 | 14.12 | 0.8125 | |
| 50 | 0.991 | 69.43 | 0.039 | 0.973 | 8.008 | 2.281 | 0.9779 | 12.53 | 0.6995 | |
| 温度 /℃ | Langmuir | Freundlich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | qm/mg·g-1 | b/L·mg-1 | R2 | KF/mg1-(1/n)·L1/n ·g-1 | n | R2 | BT/J·mol-1 | KT/L·mg-1 | ||
| 30 | 0.994 | 85.21 | 0.052 | 0.968 | 8.116 | 1.939 | 0.9688 | 14.94 | 0.9734 | |
| 40 | 0.991 | 75.74 | 0.050 | 0.966 | 7.225 | 1.973 | 0.9844 | 14.12 | 0.8125 | |
| 50 | 0.991 | 69.43 | 0.039 | 0.973 | 8.008 | 2.281 | 0.9779 | 12.53 | 0.6995 | |
| 吸附剂 | pH | 吸附剂剂量/g·L-1 | 吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| LaAlO3 | 3 | 2 | 66.50 | 本实验 |
| Mg-Al-Ce | 6 | 5 | 26.12 | [ |
| La/MA | 6 | 2 | 26.45 | [ |
| Ce-Fe | 5.5 | 0.5 | 60.97 | [ |
| TiO2-ZrO2 | 5 | 0.5 | 27.80 | [ |
| 吸附剂 | pH | 吸附剂剂量/g·L-1 | 吸附容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| LaAlO3 | 3 | 2 | 66.50 | 本实验 |
| Mg-Al-Ce | 6 | 5 | 26.12 | [ |
| La/MA | 6 | 2 | 26.45 | [ |
| Ce-Fe | 5.5 | 0.5 | 60.97 | [ |
| TiO2-ZrO2 | 5 | 0.5 | 27.80 | [ |
| 1 | LACSON Carl Francis Z, LU Mingchun, HUANG Yaohui. Fluoride-containing water: A global perspective and a pursuit to sustainable water defluoridation management—An overview[J]. Journal of Cleaner Production, 2021, 280: 124236. |
| 2 | PETERS RUDOLPH, SHORTHOUSE M. Fluoride metabolism in plants[J]. Nature, 1964, 202(4927): 21-22. |
| 3 | HE Junyong, YANG Ya, WU Zijian, et al. Review of fluoride removal from water environment by adsorption[J]. Journal of Environmental Chemical Engineering, 2020, 8(6): 104516. |
| 4 | HE Xiaodong, LI Peiyue, JI Yujie, et al. Groundwater arsenic and fluoride and associated arsenicosis and fluorosis in China: Occurrence, distribution and management[J]. Exposure and Health, 2020, 12(3): 355-368. |
| 5 | 张广瑞, 任彬, 李海松. 煤化工废水除氟及尾水脱氮除磷性能[J]. 中国环境科学, 2023, 43(4): 1655-1662. |
| ZHANG Guangrui, REN Bin, LI Haisong. Defluorination of coal chemical wastewater and nitrogen and phosphorus removal performance of tailwater[J]. China Environmental Science, 2023, 43(4): 1655-1662. | |
| 6 | 杨为森, 刘毅飞, 史丰硕, 等. 电纺La2O3-CeO2纳米纤维的除氟性能[J]. 复合材料学报, 2023, 40(6): 3385-3395. |
| YANG Weisen, LIU Yifei, SHI Fengshuo, et al. Defluoridation performance of electrospun La2O3-CeO2 nanofibers[J]. Acta Materiae Compositae Sinica, 2023, 40(6): 3385-3395. | |
| 7 | CHEN Xin, WAN Caixia, YU Rui, et al. A novel carboxylated polyacrylonitrile nanofibrous membrane with high adsorption capacity for fluoride removal from water[J]. Journal of Hazardous Materials, 2021, 411: 125113. |
| 8 | 武鑫霞, 曹占平, 苏婷, 等. Ce改性金属有机骨架材料对氟的吸附[J]. 复合材料学报, 2020, 37(10): 2636-2644. |
| WU Xinxia, CAO Zhanping, SU Ting, et al. Adsorption of Ce modified metal organic framework to fluorine[J]. Acta Materiae Compositae Sinica, 2020, 37(10): 2636-2644. | |
| 9 | LI Xilin, YU Xiaowan, LIU Ling, et al. Preparation, characterization serpentine-loaded hydroxyapatite and its simultaneous removal performance for fluoride, iron and manganese[J]. RSC Advances, 2021, 11(27): 16201-16215. |
| 10 | MUKHERJEE Arnab, ADAK Mrinal K, DHAK Prasanta, et al. A simple chemical method for the synthesis of Cu2+ engrafted MgAl2O4 nanoparticles: Efficient fluoride adsorbents, photocatalyst and latent fingerprint detection[J]. Journal of Environmental Sciences, 2020, 88: 301-315. |
| 11 | 魏永, 李贤建, 罗政博, 等. 氧化铝改性活性炭纤维电吸附除氟效能及机理[J]. 中国环境科学, 2023, 43(8): 3974-3982. |
| WEI Yong, LI Xianjian, LUO Zhengbo, et al. Efficiency and mechanism of fluoride removal by electroadsorption of alumina modified activated carbon fiber[J]. China Environmental Science, 2023, 43(8): 3974-3982. | |
| 12 | GITARI Wilson M, IZUAGIE Anthony A, GUMBO Jabulani R. Synthesis, characterization and batch assessment of groundwater fluoride removal capacity of trimetal Mg/Ce/Mn oxide-modified diatomaceous earth[J]. Arabian Journal of Chemistry, 2020, 13(1): 1-16. |
| 13 | ZHANG Bo, LIU Chengjun, LI Chunlong, et al. A novel approach for recovery of rare earths and niobium from Bayan Obo tailings[J]. Minerals Engineering, 2014, 65: 17-23. |
| 14 | AOUDJ S, KHELIFA A, DROUICHE N, et al. Removal of fluoride and turbidity from semiconductor industry wastewater by combined coagulation and electroflotation[J]. Desalination and Water Treatment, 2016, 57(39): 18398-18405. |
| 15 | HUANG Lei, YANG Zhihui, LEI Dongxue, et al. Experimental and modeling studies for adsorbing different species of fluoride using lanthanum-aluminum perovskite[J]. Chemosphere, 2021, 263: 128089. |
| 16 | LI W, ZHUO M W, SHI J L. Synthesizing nano LaAlO3 powders via co-precipitation method[J]. Materials Letters, 2004, 58(3/4): 365-368. |
| 17 | KUO Chia-Liang, WANG Chengli, CHEN Teyuan, et al. Low temperature synthesis of nanocrystalline lanthanum monoaluminate powders by chemical coprecipitation[J]. Journal of Alloys and Compounds, 2007, 440(1/2): 367-374. |
| 18 | BRYLEWSKI Tomasz, BUĆKO Mirosław M. Low-temperature synthesis of lanthanum monoaluminate powders using the co-precipitation-calcination technique[J]. Ceramics International, 2013, 39(5): 5667-5674. |
| 19 | HARON Wankassama, WISITSORAAT Anurat, WONGNAWA Sumpun. Nanostructured perovskite oxides-LaMO3 (M=Al, Co, Fe) prepared by co-precipitation method and their ethanol-sensing characteristics[J]. Ceramics International, 2017, 43(6): 5032-5040. |
| 20 | 宋立. 高分散性纳米YAG荧光粉的制备及性能研究[D]. 南京: 东南大学, 2018. |
| SONG Li. Preparation and properties of high dispersion nano-YAG phosphor[D].Nanjing: Southeast University, 2018. | |
| 21 | Yujin SIM, YOO Jihoon, Jeong-Myeong HA, et al. Oxidative coupling of methane over LaAlO3 perovskite catalysts prepared by a co-precipitation method: Effect of co-precipitation pH value[J]. Journal of Energy Chemistry, 2019, 35: 1-8. |
| 22 | WALTON Prof Richard I. Frontispiece: Perovskite oxides prepared by hydrothermal and solvothermal synthesis: A review of crystallisation, chemistry, and compositions[J]. Chemistry-A European Journal, 2020, 26(42): 9041-9069. |
| 23 | ZHANG Lianjie, CUI Meisheng, WANG Hao, et al. Effects of co-precipitation temperature on structure and properties of La and Y doped cerium zirconium mixed oxides[J]. Transactions of Nonferrous Metals Society of China, 2022, 32(2): 618-628. |
| 24 | GAYER K H, THOMPSON L C, ZAJICEK O T. The solubility of aluminum hydroxide in acidic and basic media at 25℃[J]. Canadian Journal of Chemistry, 1958, 36(9): 1268-1271. |
| 25 | LEE Gihoon, KANG Ji Yeon, YAN Ning, et al. Simple preparation method for Mg-Al hydrotalcites as base catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2016, 423: 347-355. |
| 26 | 刘文斌. Nd: YAG透明陶瓷的制备、显微结构及激光性能研究[D]. 上海: 上海交通大学, 2012. |
| LIU Wenbin. Preparation, microstructure and laser properties of Nd: YAG transparent ceramics[D]. Shanghai: Shanghai Jiao Tong University, 2012. | |
| 27 | SOLANGI Imam Bakhsh, MEMON Shahabuddin, BHANGER M I. An excellent fluoride sorption behavior of modified amberlite resin[J]. Journal of Hazardous Materials, 2010, 176(1/2/3): 186-192. |
| 28 | ISLAM Mahamudur, MISHRA Prakash Chandra, PATEL Rajkishore. Fluoride adsorption from aqueous solution by a hybrid thorium phosphate composite[J]. Chemical Engineering Journal, 2011, 166(3): 978-985. |
| 29 | WANG Aihe, ZHOU Kanggen, CHEN Wei, et al. Adsorption of fluoride by the calcium alginate embedded with Mg-Al-Ce trimetal oxides[J]. Korean Journal of Chemical Engineering, 2018, 35(8): 1636-1641. |
| 30 | HE Yuxuan, ZHANG Liming, AN Xiao, et al. Enhanced fluoride removal from water by rare earth (La and Ce) modified alumina: Adsorption isotherms, kinetics, thermodynamics and mechanism[J]. Science of the Total Environment, 2019, 688: 184-198. |
| 31 | TANG Dandan, ZHANG Gaoke. Efficient removal of fluoride by hierarchical Ce-Fe bimetal oxides adsorbent: Thermodynamics, kinetics and mechanism[J]. Chemical Engineering Journal, 2016, 283: 721-729. |
| 32 | YU Yaqin, ZHOU Zhen, DING Zhaoxia, et al. Simultaneous arsenic and fluoride removal using{201}TiO2-ZrO2: Fabrication, characterization, and mechanism[J]. Journal of Hazardous Materials, 2019, 377: 267-273. |
| [1] | ZHI Yuan, MA Jiliang, CHEN Xiaoping, LIU Daoyin, LIANG Cai. Decarbonization capability of supported Na-based CO2 adsorbents prepared by fluidized bed spray impregnation [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2961-2967. |
| [2] | MIAO Yihe, WANG Yaozu, LIU Yuhang, ZHU Xuancan, LI Jia, YU Lijun. Research progress on the improving effect of additives on supported amine adsorbents for carbon capture [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2739-2759. |
| [3] | DONG Xiaohan, TIAN Yue, SU Yi. Study on the preparation of composite adsorbent with titanium-containing blast furnace slag and chromium adsorption performance [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1552-1564. |
| [4] | DAI Hongjing, MA Xuehu, WANG Sifang. Adsorption technology and materials for the treatment of low and intermediate level radioactive wastewater [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 529-540. |
| [5] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [6] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [7] | ZHANG Xuewei, HUANG Yaji, XU Yueyang, CHENG Haoqiang, ZHU Zhicheng, LI Jinlei, DING Xueyu, WANG Sheng, ZHANG Rongchu. Adsorption characteristics of SO3 from coal flue gas by alkaline adsorbent [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3855-3864. |
| [8] | ZHAO Chongyang, ZHAO Lei, SHI Xiangwen, HUANG Jun, LI Zhiyao, SHEN Kai, ZHANG Yaping. Effect of O2/H2O/SO2 on the adsorption of PbCl2 by modified iron-rich attapulgite at high temperature [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2190-2200. |
| [9] | GUO Shuaishuai, CHEN Jinlu, JIN Liangchenglong, TAO Zui, CHEN Xiaoli, PENG Guowen. Research progress of porous aromatic frameworks based on uranium extraction from seawater [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1426-1436. |
| [10] | YE Qinhui, CHEN Hong, YU Xin, WANG Kai, YU Luying, ZENG Kejia. Preparation and resource utilization of biogas residue biochar [J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6554-6566. |
| [11] | GUO Yu, TONG Minxin, WU Hongmei. Preparation of amino-functionalized dialdehyde starch adsorbent for adsorption of Pb(Ⅱ) ions [J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6589-6599. |
| [12] | CUI Qian, WANG Annan, CHEN Zaiming, SUN Qiaoyi, WANG Baodeng, WANG Yongsheng, SUN Nannan, HU Jian, LI Jingfeng, XIONG Rihua. Preparation and performance optimization of liquefied residue-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6620-6630. |
| [13] | WU Yue, LI Xiaoyu, TAO Chunhui, ZHANG Ying, LI Yinhui, ZHANG Wenxiang, Yang Bolun, MA Heping. Adsorptive separation of NF3 using ion-modified CON material [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6076-6085. |
| [14] | WANG Yuqing, DUAN Yufeng, WANG Rui, LIU Xiaoshuo, SHEN Zhen. Experimental and kinetics analysis of ethanol-hydrated calcium-based adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6053-6063. |
| [15] | KE Yuxin, ZHU Xiaoli, SI Shaocheng, ZHANG Ting, WANG Junqiang, ZHANG Ziye. Adsorbent derived from spent bleaching earth for the synergistic removal of tetracycline and copper in wastewater [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5981-5992. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||