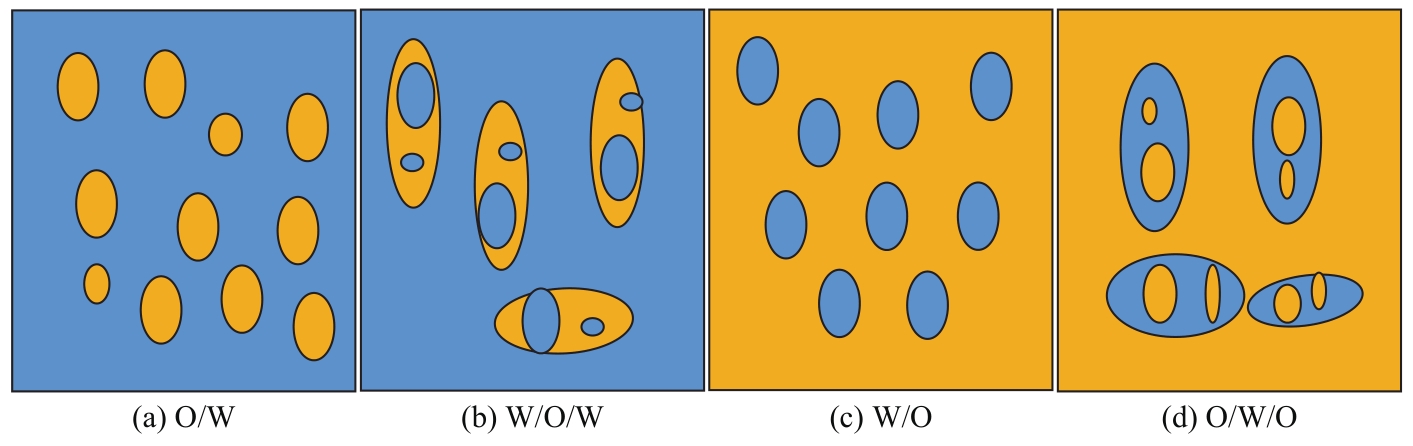
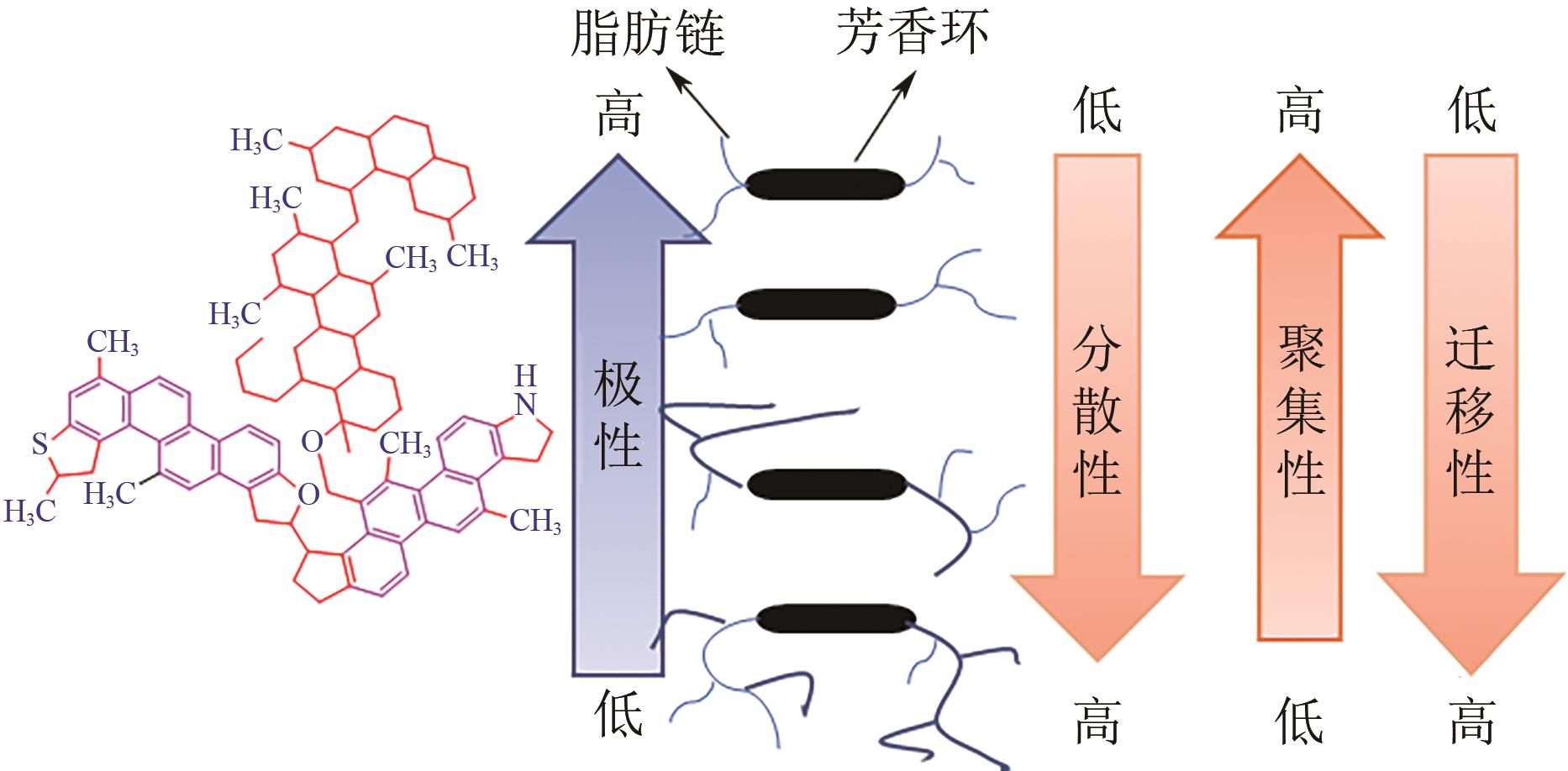
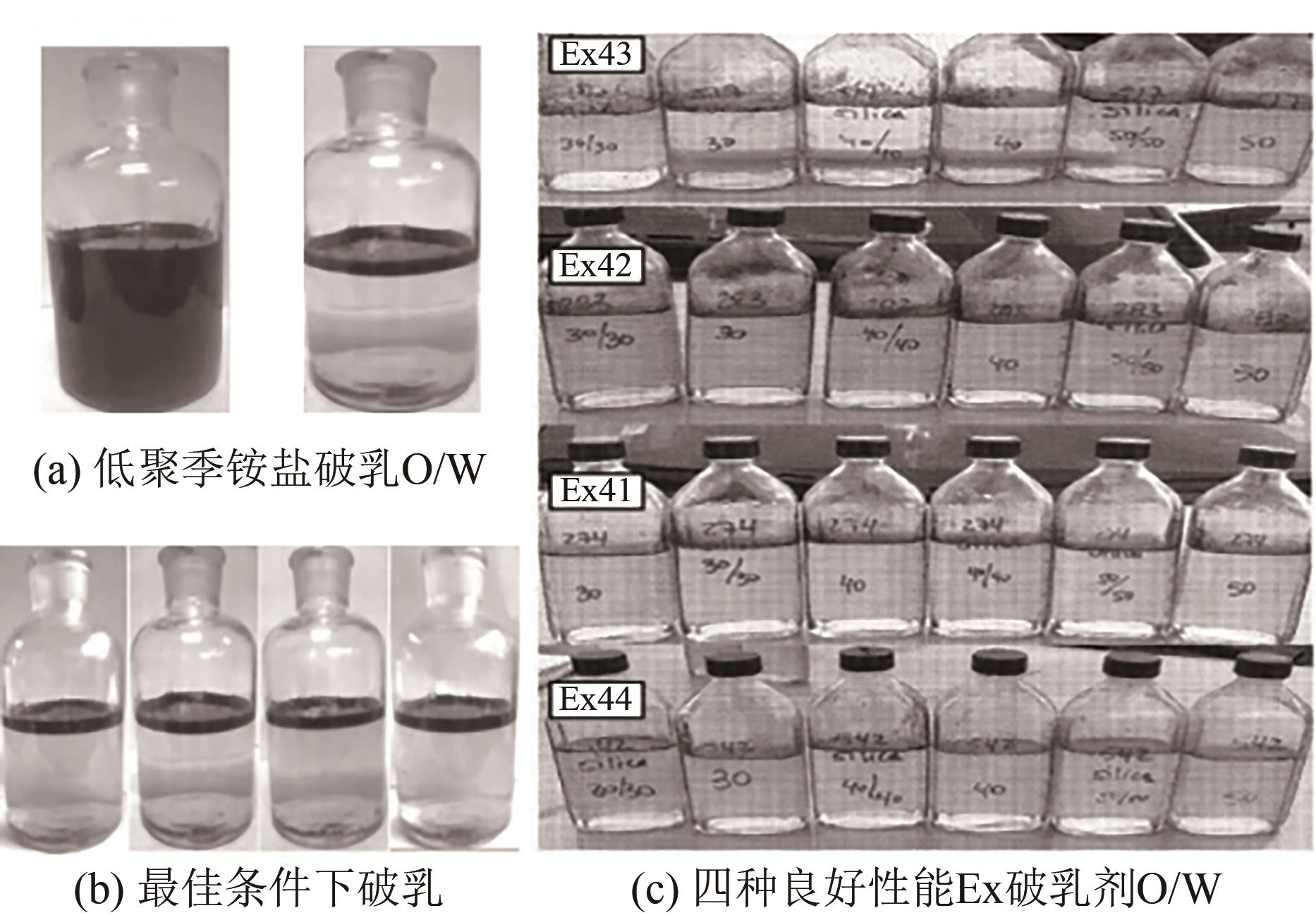
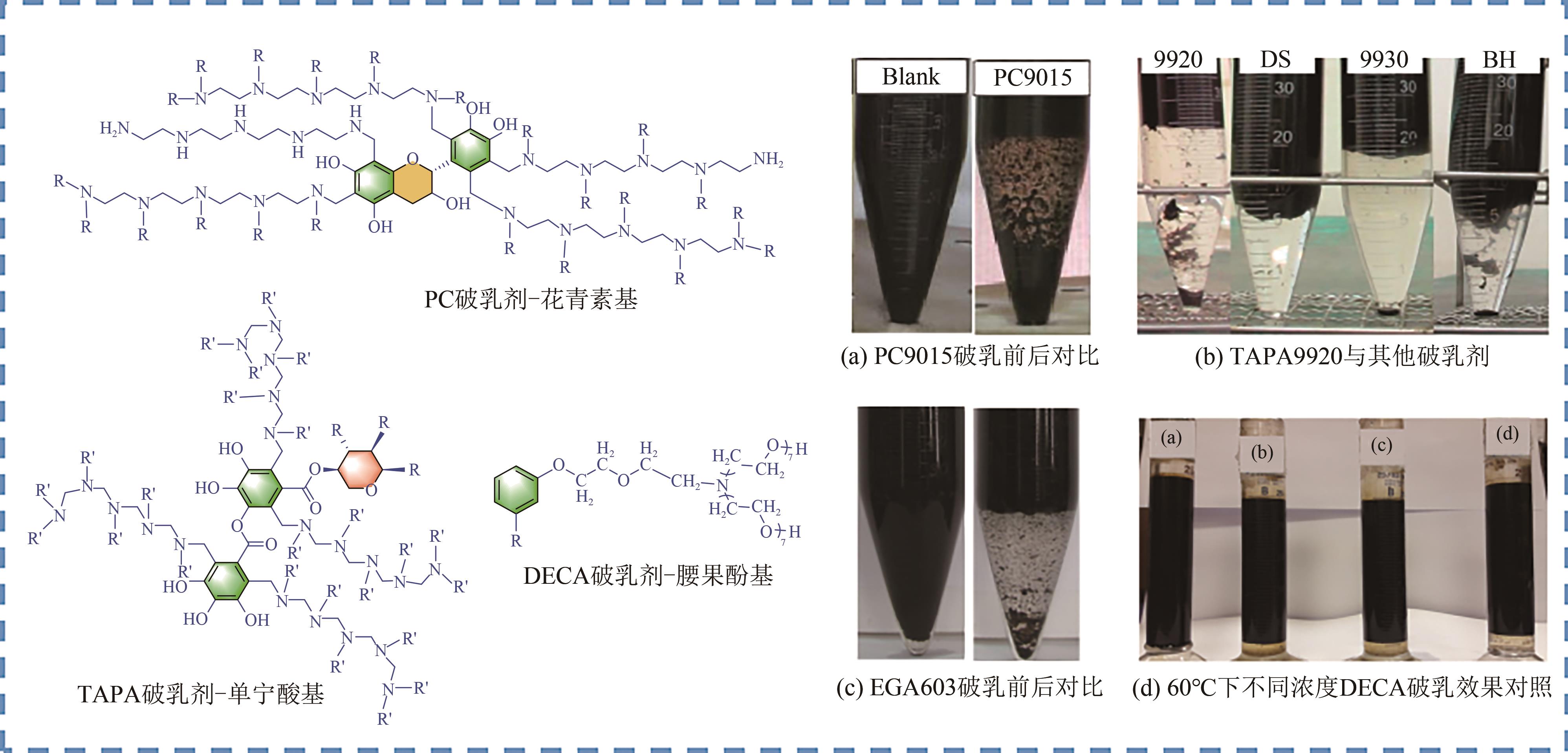
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (8): 4398-4413.DOI: 10.16085/j.issn.1000-6613.2022-1754
• Resources and environmental engineering • Previous Articles Next Articles
Research progress on highly efficient demulsifiers for complex crude oil emulsions and their applications
WU Ya1,2( ), ZHAO Dan1, FANG Rongmiao1, LI Jingyao1, CHANG Nana1, DU Chunbao1, WANG Wenzhen1,2(
), ZHAO Dan1, FANG Rongmiao1, LI Jingyao1, CHANG Nana1, DU Chunbao1, WANG Wenzhen1,2( ), SHI Jun1(
), SHI Jun1( )
)
- 1.College of Chemistry and Chemical Engineering, Xi’an Shiyou University, Xi’an 710065, Shaanxi, China
2.Shaanxi Engineering Research Center of Green Low-carbon Energy Materials and Processes, Xi’an 710065, Shaanxi, China
-
Received:2022-09-20Revised:2023-01-19Online:2023-09-19Published:2023-08-15 -
Contact:WANG Wenzhen, SHI Jun
用于复杂原油乳液的高效破乳剂开发及应用研究进展
吴亚1,2( ), 赵丹1, 方荣苗1, 李婧瑶1, 常娜娜1, 杜春保1, 王文珍1,2(
), 赵丹1, 方荣苗1, 李婧瑶1, 常娜娜1, 杜春保1, 王文珍1,2( ), 史俊1(
), 史俊1( )
)
- 1.西安石油大学化学化工学院,陕西 西安 710065
2.陕西省绿色低碳能源材料与过程工程技术研究中心,陕西 西安 710065
-
通讯作者:王文珍,史俊 -
作者简介:吴亚(1979—),女,博士,教授,研究方向为提高油田水质。E-mail:wuya@xsyu.edu.cn。 -
基金资助:国家自然科学基金(22002117);陕西省重点研发计划工业领域(2022GY-162);陕西省重点研发计划国际科技合作与交流项目(2019KW-061)
CLC Number:
Cite this article
WU Ya, ZHAO Dan, FANG Rongmiao, LI Jingyao, CHANG Nana, DU Chunbao, WANG Wenzhen, SHI Jun. Research progress on highly efficient demulsifiers for complex crude oil emulsions and their applications[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4398-4413.
吴亚, 赵丹, 方荣苗, 李婧瑶, 常娜娜, 杜春保, 王文珍, 史俊. 用于复杂原油乳液的高效破乳剂开发及应用研究进展[J]. 化工进展, 2023, 42(8): 4398-4413.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1754
| 破乳剂 | 适用油水体系类型 | DE/% | 最佳破乳环境 | 优点 | 缺点 |
|---|---|---|---|---|---|
| BP系列[ | 胜利油田 | 90以上 | 50℃,3h | 脱水效果好 | 对分子量和破乳温度要求高,否则破乳效果不明显,对环境污染较大 |
| AP系列[ | 渤海油田、东海油田 | 90 | 1.25%的水分散体系破乳剂用量25mg/L | 对海洋原油具有脱水速度快、油水界面齐、脱出水清 | |
| SD破乳剂[ | 塔河油田重质原油 | 89 | 破乳剂用量100mg/L,温度为70℃ | — | |
| DS-06/DL11正反破乳剂复配[ | 呼和浩特炼油厂含油污泥 | 95 | 180mg/L,30∶10复配 | 专一性强,工业应用效果明显 | |
| DS-1复配型[ | 大庆油田北二联合站高机械杂质含量污油 | 97 | 4.5h,50℃ | — |
| 破乳剂 | 适用油水体系类型 | DE/% | 最佳破乳环境 | 优点 | 缺点 |
|---|---|---|---|---|---|
| BP系列[ | 胜利油田 | 90以上 | 50℃,3h | 脱水效果好 | 对分子量和破乳温度要求高,否则破乳效果不明显,对环境污染较大 |
| AP系列[ | 渤海油田、东海油田 | 90 | 1.25%的水分散体系破乳剂用量25mg/L | 对海洋原油具有脱水速度快、油水界面齐、脱出水清 | |
| SD破乳剂[ | 塔河油田重质原油 | 89 | 破乳剂用量100mg/L,温度为70℃ | — | |
| DS-06/DL11正反破乳剂复配[ | 呼和浩特炼油厂含油污泥 | 95 | 180mg/L,30∶10复配 | 专一性强,工业应用效果明显 | |
| DS-1复配型[ | 大庆油田北二联合站高机械杂质含量污油 | 97 | 4.5h,50℃ | — |
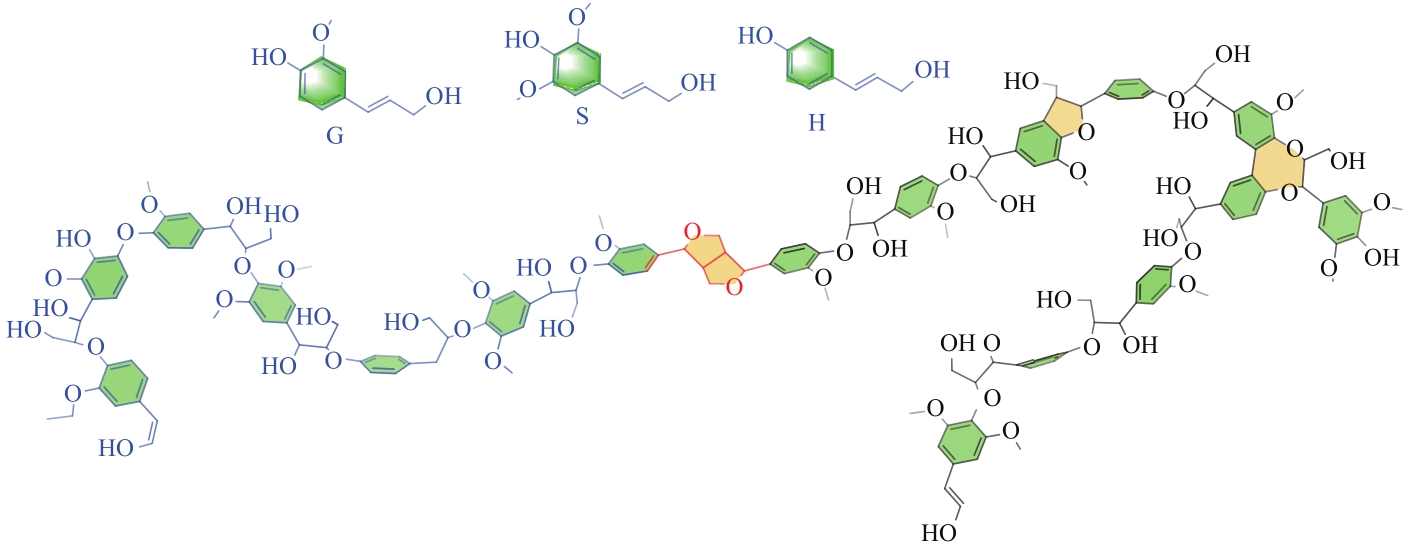
| 生物质破乳剂原料 | 生物质破乳剂 | 适用乳液类型 | DE/% | 最佳破乳环境 | 优点 | 缺点 |
|---|---|---|---|---|---|---|
| 花青素[ | PC9015 | 渤海油田老化油乳液 | 84.6 | 70℃,2h,100mg/L | 脱水性能好,实用性较强,适用于海上平台的原油和老化油破乳,以天然多酚为原料,更为经济、绿色 油水界面清晰,无挂壁,取代了用于制备常规石油衍生的壬基酚表面活性剂 | 挂壁严重 |
| 表没食子儿茶素没食子酸酯[ | EGA603 | 渤海油田老化原油乳液 | 94.3 | 70℃,100min,100mg/L | 挂壁较轻 | |
| 单宁酸[ | TAPA9920 | 南海油田老化油乳液 | 97.9 | 70℃,40min,100mg/L | 合成困难 | |
| 腰果酚[ | DECA | 阿拉伯重质原油 | 100 | 60℃,40min,100mg/L | 合成困难,尚在实验室阶段 | |
| 木质素[ | 木质素和二氧化硅的高效膜 | O/W体系 | 98.6 | 与滤纸表面完全接触。一旦有色油被完全过滤掉,分离过程就算作结束 | 效果明显,分离简单 | |
| 荷叶[ | HLLF | 长庆油田 | 88.17 | 1000mg/L在70℃下持续90min | 油水界面清晰,水质清亮 | 适用范围较窄,DEMLOCS较其他相似产品剂量颇高 |
| 稻壳[ | RHC | W/O体系 | 96.99 | 600mg/L,70℃,80min | 具有较宽的pH范围和良好的耐盐性 | 适用范围较窄,DEMLOCS较其他相似产品剂量颇高 |
| 椰子提取物 | DEMLOCS | 老化油乳液 | 88 | 45℃,24h,2000mg/L | 温度适宜,水热法合成步骤简单 |
| 生物质破乳剂原料 | 生物质破乳剂 | 适用乳液类型 | DE/% | 最佳破乳环境 | 优点 | 缺点 |
|---|---|---|---|---|---|---|
| 花青素[ | PC9015 | 渤海油田老化油乳液 | 84.6 | 70℃,2h,100mg/L | 脱水性能好,实用性较强,适用于海上平台的原油和老化油破乳,以天然多酚为原料,更为经济、绿色 油水界面清晰,无挂壁,取代了用于制备常规石油衍生的壬基酚表面活性剂 | 挂壁严重 |
| 表没食子儿茶素没食子酸酯[ | EGA603 | 渤海油田老化原油乳液 | 94.3 | 70℃,100min,100mg/L | 挂壁较轻 | |
| 单宁酸[ | TAPA9920 | 南海油田老化油乳液 | 97.9 | 70℃,40min,100mg/L | 合成困难 | |
| 腰果酚[ | DECA | 阿拉伯重质原油 | 100 | 60℃,40min,100mg/L | 合成困难,尚在实验室阶段 | |
| 木质素[ | 木质素和二氧化硅的高效膜 | O/W体系 | 98.6 | 与滤纸表面完全接触。一旦有色油被完全过滤掉,分离过程就算作结束 | 效果明显,分离简单 | |
| 荷叶[ | HLLF | 长庆油田 | 88.17 | 1000mg/L在70℃下持续90min | 油水界面清晰,水质清亮 | 适用范围较窄,DEMLOCS较其他相似产品剂量颇高 |
| 稻壳[ | RHC | W/O体系 | 96.99 | 600mg/L,70℃,80min | 具有较宽的pH范围和良好的耐盐性 | 适用范围较窄,DEMLOCS较其他相似产品剂量颇高 |
| 椰子提取物 | DEMLOCS | 老化油乳液 | 88 | 45℃,24h,2000mg/L | 温度适宜,水热法合成步骤简单 |
| 性能 | 离子液体 | 传统破乳剂(表面活性剂) |
|---|---|---|
| 界面张力 | 表面活性离子液体可以将界面张力降低到10-2mN/m,有必要提高离子液体的表面活性,并注意界面张力与盐度的协同作用 | 可以降低IFT达到10-4~10-3mN/m,显示了它们在油水分离应用中的有效性 |
| 黏度 | 离子液体的黏度可以通过支化的变化来调节 | 在水溶液中,它们无法产生更高的黏度 |
| 稳定性 | 离子液体高温下是稳定的,可以长时间储存而不会分解 | 表面活性剂在高温下不稳定 |
| 毒性 | 离子液体毒性较小且环保 | 部分烷基酚表面活性剂毒性大,无法达到降解要求 |
| 可回收性 | 回收离子液体的方法有很多种,如液液萃取、蒸馏、吸附、结晶、力场分离和膜分离等[ | 不可回收 |
| 性能 | 离子液体 | 传统破乳剂(表面活性剂) |
|---|---|---|
| 界面张力 | 表面活性离子液体可以将界面张力降低到10-2mN/m,有必要提高离子液体的表面活性,并注意界面张力与盐度的协同作用 | 可以降低IFT达到10-4~10-3mN/m,显示了它们在油水分离应用中的有效性 |
| 黏度 | 离子液体的黏度可以通过支化的变化来调节 | 在水溶液中,它们无法产生更高的黏度 |
| 稳定性 | 离子液体高温下是稳定的,可以长时间储存而不会分解 | 表面活性剂在高温下不稳定 |
| 毒性 | 离子液体毒性较小且环保 | 部分烷基酚表面活性剂毒性大,无法达到降解要求 |
| 可回收性 | 回收离子液体的方法有很多种,如液液萃取、蒸馏、吸附、结晶、力场分离和膜分离等[ | 不可回收 |
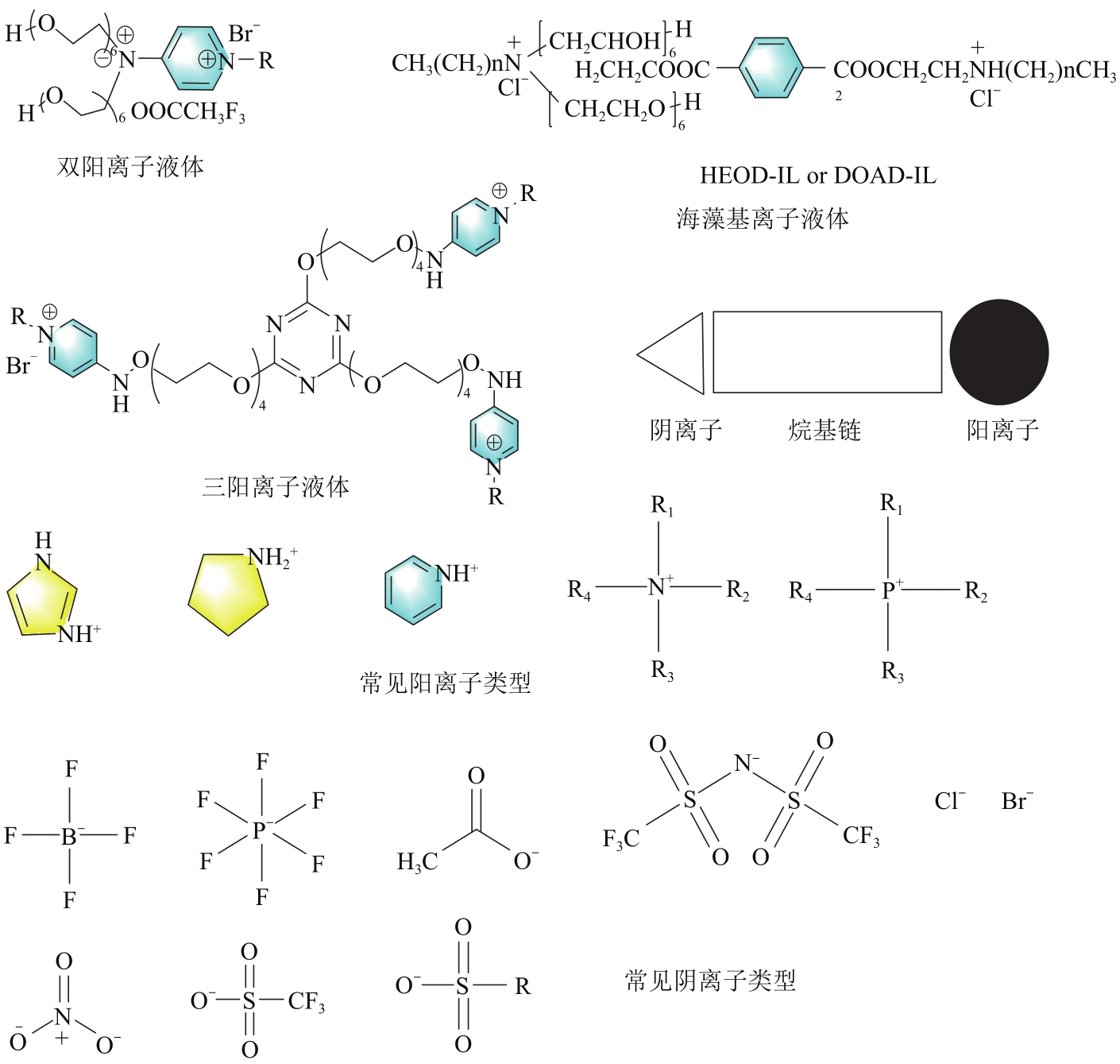
| 离子液体 | 阳离子 | 阴离子 | 乳液 类型 | 剂量/mg·L-1 | DE/% | 界面张力 降低 | 结论 |
|---|---|---|---|---|---|---|---|
| C n mim NTf2(n=10、12、14) | 咪唑啉 | 双(三氟甲基 磺酰基)亚胺 | SW/O | 100~3500 | 93.6~100 | 77~95 | 对于亲水性离子液体,增加离子液体的剂量和烷基阳离子链长度导致聚集并导致破乳不良以及增加IFT[ |
| C n mim PF6(n=10、12、14) | 六氟磷酸 | 500~3500 | 71.25~86.25 | 54~81 | |||
| C n mim Cl(n=10、12、14) | 氯离子 | 500~3500 | 76.25~93.75 | 64~80 | |||
| 三阳离子液体 | |||||||
| TNPy | 氨基吡啶 | 溴离子 | W/O | 250~1000 | 100 | 24 | 研究了烷基链长度、破乳剂剂量和RSN值对破乳活性的影响。结果表明,与含有十六烷基链的TCPyILs相比,壬基和十四烷基链的TCPyILs具有更好和更高的破乳效率。TNPy和TTPyIL对W/O(50/50、30/70和10/90,v/v)的破乳效率即使在低浓度下也达到100%[ |
| TTPy | |||||||
| W/O | 250~1000 | 100 | 26.5 | ||||
| TCPy | |||||||
| W/O | 500 | 100 | 26 | ||||
| 绿色环保的ILs | |||||||
| 海藻基 | 铵 | 溴离子 | SW/O | 1000 | 100 | — | BH-ALG和BN-ALG都表现出良好的降低界面张力的能力。在这两种PIL中,BH-ALG的表面活性高于BN-ALG,这可能是由于与BN-ALG相比,其烷基链更长,因此具有更高的疏水性[ |
| 松香酸(AA) | 咪唑 | 碘离子 | SW/O | 250~1000 | 100 | — | 在原油与盐水体积比为90∶10时,两种AILs的脱水率均达到100%,这可以称为由于存在聚乙二酸酯环而增加其疏水性[ |
| 废弃塑料[ | 铵 | 氯离子 | W/O | 1000 | 94 | 34.5 | PET废物的糖酵解产生BHET,其与氯化亚砜反应形成BCET,然后与HEOD和DOAD进行季铵化,分别形成相应的两亲性GIL、HEOD-IL和DOAD-IL。HEOD-IL的CMC低于DOAD-IL,两种两亲性GIL中烷基链的存在有助于它们在连续相中的扩散,以取代刚性沥青质薄膜 |
| 聚离子液体修饰的磁性纳米复合材料 | |||||||
| 乙烯基咪唑 | 溴离子 | W/O | 1000 | 99.9±0.3 | 18±0.1 | 对于W/O乳液,1000μg/L的破乳剂,在70℃下2h后获得了(99.9±0.3)%的破乳效率。破乳后,破乳剂的界面张力从(20.0±0.1)mN/m降低到(1.9±0.1)mN/m,证明了其破乳率高。第一次破乳循环后,通破乳剂过外部磁铁从容器中取出,并重新用于下一个破乳循环。重复使用4个循环后,其破乳效率达到89%[ | |
| 离子液体 | 阳离子 | 阴离子 | 乳液 类型 | 剂量/mg·L-1 | DE/% | 界面张力 降低 | 结论 |
|---|---|---|---|---|---|---|---|
| C n mim NTf2(n=10、12、14) | 咪唑啉 | 双(三氟甲基 磺酰基)亚胺 | SW/O | 100~3500 | 93.6~100 | 77~95 | 对于亲水性离子液体,增加离子液体的剂量和烷基阳离子链长度导致聚集并导致破乳不良以及增加IFT[ |
| C n mim PF6(n=10、12、14) | 六氟磷酸 | 500~3500 | 71.25~86.25 | 54~81 | |||
| C n mim Cl(n=10、12、14) | 氯离子 | 500~3500 | 76.25~93.75 | 64~80 | |||
| 三阳离子液体 | |||||||
| TNPy | 氨基吡啶 | 溴离子 | W/O | 250~1000 | 100 | 24 | 研究了烷基链长度、破乳剂剂量和RSN值对破乳活性的影响。结果表明,与含有十六烷基链的TCPyILs相比,壬基和十四烷基链的TCPyILs具有更好和更高的破乳效率。TNPy和TTPyIL对W/O(50/50、30/70和10/90,v/v)的破乳效率即使在低浓度下也达到100%[ |
| TTPy | |||||||
| W/O | 250~1000 | 100 | 26.5 | ||||
| TCPy | |||||||
| W/O | 500 | 100 | 26 | ||||
| 绿色环保的ILs | |||||||
| 海藻基 | 铵 | 溴离子 | SW/O | 1000 | 100 | — | BH-ALG和BN-ALG都表现出良好的降低界面张力的能力。在这两种PIL中,BH-ALG的表面活性高于BN-ALG,这可能是由于与BN-ALG相比,其烷基链更长,因此具有更高的疏水性[ |
| 松香酸(AA) | 咪唑 | 碘离子 | SW/O | 250~1000 | 100 | — | 在原油与盐水体积比为90∶10时,两种AILs的脱水率均达到100%,这可以称为由于存在聚乙二酸酯环而增加其疏水性[ |
| 废弃塑料[ | 铵 | 氯离子 | W/O | 1000 | 94 | 34.5 | PET废物的糖酵解产生BHET,其与氯化亚砜反应形成BCET,然后与HEOD和DOAD进行季铵化,分别形成相应的两亲性GIL、HEOD-IL和DOAD-IL。HEOD-IL的CMC低于DOAD-IL,两种两亲性GIL中烷基链的存在有助于它们在连续相中的扩散,以取代刚性沥青质薄膜 |
| 聚离子液体修饰的磁性纳米复合材料 | |||||||
| 乙烯基咪唑 | 溴离子 | W/O | 1000 | 99.9±0.3 | 18±0.1 | 对于W/O乳液,1000μg/L的破乳剂,在70℃下2h后获得了(99.9±0.3)%的破乳效率。破乳后,破乳剂的界面张力从(20.0±0.1)mN/m降低到(1.9±0.1)mN/m,证明了其破乳率高。第一次破乳循环后,通破乳剂过外部磁铁从容器中取出,并重新用于下一个破乳循环。重复使用4个循环后,其破乳效率达到89%[ | |
| 1 | SOUSA A M, PEREIRA M J, MATOS H A. Oil-in-water and water-in-oil emulsions formation and demulsification[J]. Journal of Petroleum Science and Engineering, 2022, 210: 110041. |
| 2 | KOVALEVA M A, KURBATOVA A D, LYSYANNIKOV A V, et al. Reasons for formation of stable intermediate layer water-in-oil emulsions in tanks[J]. IOP Conference Series: Earth and Environmental Science, 2019, 315(6): 062012. |
| 3 | FAIZULLAYEV Saidulla, ADILBEKOVA Akbota, KUJAWSKI Wojciech, et al. Recent demulsification methods of crude oil emulsions—Brief review[J]. Journal of Petroleum Science and Engineering, 2022, 215: 110643. |
| 4 | SOUSA A M, PEREIRA M J, MATOS H A. Oil-in-water and water-in-oil emulsions formation and demulsification[J]. Journal of Petroleum Science and Engineering, 2022, 210: 110041. |
| 5 | 张涛, 袁宏强, 戴俊峰, 等. 油田老化油处理技术现状[J]. 石油化工应用, 2021, 40(3): 1-5. |
| ZHANG Tao, YUAN Hongqiang, DAI Junfeng, et al. Situation of aging oil treatment technologies in oilfield[J]. Petrochemical Industry Application, 2021, 40(3): 1-5. | |
| 6 | 韩创辉, 刘贵宾, 连宇博, 等. 长庆油田H作业区老化油的处理研究[J]. 石油化工应用, 2020, 39(8): 60-64. |
| HAN Chuanghui, LIU Guibin, LIAN Yubo, et al. Study on treatment of aging oil in operation area H of Changqing oilfield[J]. Petrochemical Industry Application, 2020, 39(8): 60-64. | |
| 7 | 王伟波, 苗林, 王君鹏, 等. 一种老化油用低温破乳剂的研究与应用[J]. 石油化工应用, 2019, 38(2): 70-75. |
| WANG Weibo, MIAO Lin, WANG Junpeng, et al. Experimental study on a low temperature demulsifier for aging oil[J]. Petrochemical Industry Application, 2019, 38(2): 70-75. | |
| 8 | SALAGER J L, MARQUEZ R, DELGADO-LINARES J G, et al. Fundamental basis for action of a chemical demulsifier revisited after 30 years: HLDN as the primary criterion for water-in-crude oil emulsion breaking[J]. Energy & Fuels, 2022, 36(2): 711-730. |
| 9 | SHEHZAD F, HUSSEIN I A, KAMAL M S, et al. Polymeric surfactants and emerging alternatives used in the demulsification of produced water: A review[J]. Polymer Reviews, 2018, 58(1): 63-101. |
| 10 | LIU Shasha, ZHANG Yanru, YAN Haijun, et al. Laboratory study on active aging oil plugging agent[J]. IOP Conference Series: Materials Science and Engineering, 2020, 729(1): 012024. |
| 11 | LIU Jia, ZHANG Yixuan, PENG Kaiming, et al. A review of the interfacial stability mechanism of aging oily sludge: Heavy components, inorganic particles, and their synergism[J]. Journal of Hazardous Materials, 2021, 415: 125624. |
| 12 | UMAR A A, SAAID I B, SULAIMON A A, et al. A review of petroleum emulsions and recent progress on water-in-crude oil emulsions stabilized by natural surfactants and solids[J]. Journal of Petroleum Science and Engineering, 2018, 165: 673-690. |
| 13 | RAO Feng, LIU Qi. Froth treatment in athabasca oil sands bitumen recovery process: A review[J]. Energy & Fuels, 2013, 27(12): 7199-7207. |
| 14 | ABED S, ABDURAHMAN N, YUNUS R, et al. Oil emulsions and the different recent demulsification techniques in the petroleum industry—A review[J]. IOP Conference Series: Materials Science and Engineering, 2019, 702(1): 012060. |
| 15 | ZHAO M W, HE H H, DAI C L, et al. Enhanced oil recovery study of a new mobility control system on the dynamic imbibition in a tight oil fracture network model[J]. Energy & Fuels, 2018, 32(3): 2908-2915. |
| 16 | XU Yuming, DABROS Tadeusz, HAMZA Hassan. Study on the mechanism of foaming from bitumen froth treatment tailings[J]. Journal of Dispersion Science and Technology, 2007, 28(3): 413-418. |
| 17 | MA Jun, YAO Mengqin, YANG Yongli, et al. Comprehensive review on stability and demulsification of unconventional heavy oil-water emulsions[J]. Journal of Molecular Liquids, 2022, 350: 118510. |
| 18 | ABDULREDHA M M, SITI A H, LUQMAN C A. Overview on petroleum emulsions, formation, influence and demulsification treatment techniques[J]. Arabian Journal of Chemistry, 2020, 13(1): 3403-3428. |
| 19 | CHACÓN-PATIÑO M L, BLANCO-TIRADO C, ORREGO-RUIZ J A, et al. High resolution mass spectrometric view of asphaltene-SiO2 interactions[J]. Energy & Fuels, 2015, 29(3): 1323-1331. |
| 20 | YANG Fan, TCHOUKOV Plamen, DETTMAN Heather, et al. Asphaltene subfractions responsible for stabilizing water-in-crude oil emulsions. part 2: Molecular representations and molecular dynamics simulations[J]. Energy & Fuels, 2015, 29(8): 4783-4794. |
| 21 | WANG Duo, YANG Diling, HUANG Charley, et al. Stabilization mechanism and chemical demulsification of water-in-oil and oil-in-water emulsions in petroleum industry: A review[J]. Fuel, 2021, 286: 119390. |
| 22 | LIU Daiwei, LI Chuanxian, ZHANG Xiaoping, et al. Polarity effects of asphaltene subfractions on the stability and interfacial properties of water-in-model oil emulsions[J]. Fuel, 2020, 269: 117450. |
| 23 | 魏学福, 曲东江, 高在海, 等. AP系列破乳剂在海洋油田的应用[J]. 日用化学品科学, 2000, 23(S1): 133-135. |
| WEI Xuefu, QU Dongjiang, GAO Zaihai, et al. The application of AP series demulsifier in offshore oilfield[J]. Detergent & Cosmetics, 2000, 23(S1): 133-135. | |
| 24 | 彭柏群, 张瑞泉, 宋辉. 原油脱水站沉降罐上部污油破乳剂的研制[J]. 油气田地面工程, 2010, 29(3): 17-18. |
| PENG Baiqun, ZHANG Ruiquan, SONG Hui. Development of demulsifier for dirty oil in the upper part of settling tank of crude oil dehydration station[J]. Oil-Gasfield Surface Engineering, 2010, 29(3): 17-18. | |
| 25 | ZHANG Zhiqing, XU G Y, WANG F, et al. Characterization and demulsification of poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) copolymers[J]. Journal of Colloid and Interface Science, 2004, 277(2): 464-470. |
| 26 | 任广欣, 周诗杰, 李堆, 等. 重质原油抗乳化破乳剂的研制及联合应用研究[J]. 化工时刊, 2021, 35(7): 20-25, 54. |
| REN Guangxin, ZHOU Shijie, LI Dui, et al. Research on development and joint application of demulsifying demulsifier for heavy crude oil[J]. Chemical Industry Times, 2021, 35(7): 20-25, 54. | |
| 27 | 刘晓兵. 正反两项破乳剂对污油中乳化油作用[J]. 内蒙古环境保护, 1999, 11(1): 37-39. |
| LIU Xiaobing. The function of the positive and negative emulsion breaker to the emulsified oil of polluted oil[J]. Inner Mongolia Environmental Protection, 1999, 11(1): 37-39. | |
| 28 | WANG Sanyi, AXCELL Eric, BOSCH Ron, et al. Effects of chemical application on antifouling in steam-assisted gravity drainage operations[J]. Energy & Fuels, 2005, 19(4): 1425-1429. |
| 29 | 吴亚, 陈世军, 陈刚, 等. 低聚季铵盐对聚驱采出水包油乳状液破乳机理[J]. 湖南大学学报(自然科学版), 2016, 43(6): 117-123. |
| WU Ya, CHEN Shijun, CHEN Gang, et al. Demulsification mechanism of O/W emulsion containing polymer using oligomeric quaternary ammonium salt[J]. Journal of Hunan University (Natural Sciences), 2016, 43(6): 117-123. | |
| 30 | OSNESS K A, DIAZ C J. Colloidal silica addition to promote the separation of oil from water EP2956222A4[Z]. [2016-10-12]. |
| 31 | 徐家业, 张科良. 破乳剂研发的若干新进展[J]. 化工技术与开发, 2021, 50(9): 54-59. |
| XU Jiaye, ZHANG Keliang. Several new progress of demulsifier research and development[J]. Technology & Development of Chemical Industry, 2021, 50(9): 54-59. | |
| 32 | HAO Li, JIANG Bin, ZHANG Luhong, et al. Efficient demulsification of diesel-in-water emulsions by different structural dendrimer-based demulsifiers[J]. Industrial & Engineering Chemistry Research, 2016, 55(6): 1748-1759. |
| 33 | BI Yangang, LI Wusong, LIU Congcong, et al. Dendrimer-based demulsifiers for polymer flooding oil-in-water emulsions[J]. Energy & Fuels, 2017, 31(5): 5395-5401. |
| 34 | PENSINI Erica, HARBOTTLE David, YANG Fan, et al. Demulsification mechanism of asphaltene-stabilized water-in-oil emulsions by a polymeric ethylene oxide-propylene oxide demulsifier[J]. Energy & Fuels, 2014, 28(11): 6760-6771. |
| 35 | HERNÁNDEZ E I, CASTRO-SOTELO L V, AVENDAÑO-GÓMEZ J R, et al. Synthesis, characterization, and evaluation of petroleum demulsifiers of multibranched block copolymers[J]. Energy & Fuels, 2016, 30(7): 5363-5378. |
| 36 | SQUICCIARINI M. Reverse emulsion breaker polymers US09260545B1[Z]. [2016-02-16]. |
| 37 | FUENTES J V, ZAMORA E B, LI Z L, et al. Alkylacrylic-carboxyalkylacrylic random bipolymers as demulsifiers for heavy crude oils[J]. Separation and Purification Technology, 2021, 256: 117850. |
| 38 | WANG Chengjie, FANG Shenwen, DUAN Ming, et al. Synthesis and evaluation of demulsifiers with polyethyleneimine as accepter for treating crude oil emulsions[J]. Polymers for Advanced Technologies, 2015, 26(5): 442-448. |
| 39 | AN Shuguo, LI Zhongwei, CHEN Hao, et al. Proanthocyanidin-based polyether demulsifiers for the treatment of aging oil emulsions[J]. Energy & Fuels, 2020, 34(5): 5788-5797. |
| 40 | LI Zhongwei, GENG Hongkun, WANG Xiujun, et al. Noval tannic acid-based polyether as an effective demulsifier for water-in-aging crude oil emulsions[J]. Chemical Engineering Journal, 2018, 354: 1110-1119. |
| 41 | WANG Chengyu, AN Shuguo, LI Zhongwei, et al. Novel epigallocatechin gallate-based polyether surfactants: Synthesis, characterization and demulsification properties[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 623: 126757. |
| 42 | BALACHANDRAN V S, JADHAV S R, VEMULA P K, et al. Recent advances in cardanol chemistry in a nutshell: From a nut to nanomaterials[J]. Chemical Society Reviews, 2013, 42(2): 427-438. |
| 43 | LI Zhongwei, AN Shuguo, LIU Yafan, et al. Practical modification of tannic acid polyether demulsifier and its highly efficient demulsification for water-in-aging crude oil emulsions[J]. ACS Omega, 2019, 4(24): 20697-20707. |
| 44 | ATTA A M, ABDULLAH M M, AL-LOHEDAN H A, et al. Demulsification of heavy crude oil using new nonionic cardanol surfactants[J]. Journal of Molecular Liquids, 2018, 252: 311-320. |
| 45 | BERTELLA S, LUTERBACHER J S. Lignin functionalization for the production of novel materials[J]. Trends in Chemistry, 2020, 2(5): 440-453. |
| 46 | ZHANG Yuqing, ZHANG Yiwen, CAO Qiping, et al. Novel porous oil-water separation material with super-hydrophobicity and super-oleophilicity prepared from beeswax, lignin, and cotton[J]. Science of the Total Environment, 2020, 706: 135807. |
| 47 | PADILHA C E, NOGUEIRA C, MATIAS S C, et al. Fabrication of hollow polymer microcapsules and removal of emulsified oil from aqueous environment using soda lignin nanoparticles[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 603: 125260. |
| 48 | GAO Zhiwen, LANG Xueling, CHEN Shuang, et al. Mini-review on the synthesis of lignin-based phenolic resin[J]. Energy & Fuels, 2021, 35(22): 18385-18395. |
| 49 | ADRIAN Moreno, LIU Jinrong, ROBIN Gueret, et al. Unravelling the hydration barrier of lignin oleate nanoparticles for acid- and base-catalyzed functionalization in dispersion state[J]. Angewandte Chemie (International Ed in English), 2021, 60(38): 20897-20905. |
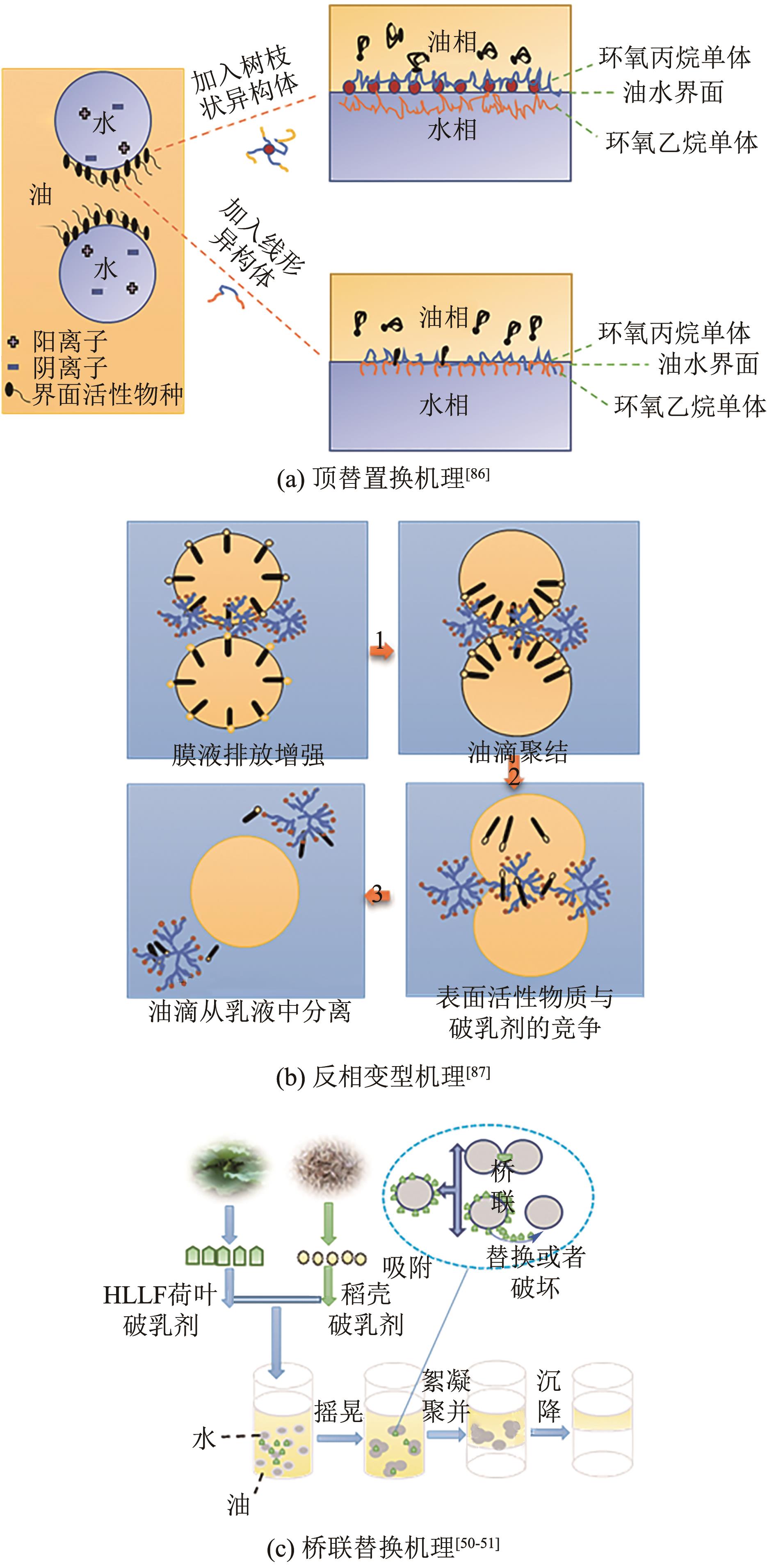
| 50 | YE Fan, MI Yuanzhu, LIU Huanyu, et al. Demulsification of water-in-crude oil emulsion using natural lotus leaf treated via a simple hydrothermal process[J]. Fuel, 2021, 295: 120596. |
| 51 | YE Fan, ZHANG Zejun, AO Yiling, et al. Demulsification of water-in-crude oil emulsion driven by a carbonaceous demulsifier from natural rice husks[J]. Chemosphere, 2022, 288: 132656. |
| 52 | DE JESUS S S, MACIEL F R. Are ionic liquids eco-friendly?[J]. Renewable and Sustainable Energy Reviews, 2022, 157: 112039. |
| 53 | NAHID Hassanshahi, HU Guangji, LI Jianbing. Application of ionic liquids for chemical demulsification: A review[J]. Molecules, 2020, 25(21): 4915. |
| 54 | KURTOĞLU-ÖZTULUM S F, JALAL A, UZUN A. Thermal stability limits of imidazolium, piperidinium, pyridinium, and pyrrolidinium ionic liquids immobilized on metal oxides[J]. Journal of Molecular Liquids, 2022, 363: 119804. |
| 55 | EMAD AL M S. Experimental study of use of ionic liquids in enhanced oil recovery[J]. Journal of Petroleum & Environmental Biotechnology, 2013, 4(6): 1-7. |
| 56 | PILLAI Prathibha, MAITI Moumita, MANDAL Ajay. Mini-review on recent advances in the application of surface-active ionic liquids: Petroleum industry perspective[J]. Energy & Fuels, 2022, 36(15): 7925-7939. |
| 57 | SAKTHIVEL S, GARDAS R L, SANGWAI J S. Effect of alkyl ammonium ionic liquids on the interfacial tension of the crude oil-water system and their use for the enhanced oil recovery using ionic liquid-polymer flooding[J]. Energy & Fuels, 2016, 30(3): 2514-2523. |
| 58 | HEZAVE A Z, DOROSTKAR S, AYATOLLAHI S, et al. Dynamic interfacial tension behavior between heavy crude oil and ionic liquid solution (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl]+ distilled or saline water/heavy crude oil)) as a new surfactant[J]. Journal of Molecular Liquids, 2013, 187: 83-89. |
| 59 | MIDTTUN Atle, KHANIEVA Marina, Magne LIA, et al. The greening of the European petroleum industry[J]. Energy Policy, 2022, 167: 112964. |
| 60 | ATTA A M, AL-LOHEDAN H A, EZZAT A O. Synthesis and application of geminal dicationic ionic liquids and poly (ionic liquids) combined imidazolium and pyridinium cations as demulsifiers for petroleum crude oil saline water emulsions[J]. Journal of Molecular Liquids, 2021, 325: 115264.[LinkOut] |
| 61 | HAZRATI N, MIRAN B A, ABDOUSS M. Demulsification of water in crude oil emulsion using long chain imidazolium ionic liquids and optimization of parameters[J]. Fuel, 2018, 229: 126-134. |
| 62 | EZZAT A O, ATTA A M, AL-LOHEDAN H A. Demulsification of stable seawater/Arabian heavy crude oil emulsions using star-like tricationic pyridinium ionic liquids[J]. Fuel, 2021, 304: 121436. |
| 63 | ABDULLAH M M, AL-LOHEDAN H A. Alginate-based poly ionic liquids for the efficient demulsification of water in heavy crude oil emulsions[J]. Fuel, 2022, 320: 123949. |
| 64 | ABDULLAH M M, EZZAT A O, AL-LOHEDAN H A, et al. New amphiphilic ionic liquids for the demulsification of water-in-heavy crude oil emulsion[J]. Molecules (Basel, Switzerland), 2022, 27(10): 3238. |
| 65 | ABDULLAH M M, AL-LOHEDAN H A. Novel amphiphilic gemini ionic liquids based on consumed polyethylene terephthalate as demulsifiers for Arabian heavy crude oil[J]. Fuel, 2020, 266: 117057. |
| 66 | AHMADI Leila, AHMADI Ebrahim, MOHAMADNIA Zahra. Demulsification of water in crude oil emulsions through magnetic nanocomposites decorated with poly(ionic liquid)S[J]. Journal of Molecular Liquids, 2022, 357: 119162. |
| 67 | LIANG Huan, ESMAEILI Hossein. Application of nanomaterials for demulsification of oily wastewater: A review study[J]. Environmental Technology & Innovation, 2021, 22: 101498. |
| 68 | 陈依文, 王睿, 文婕, 等. 纳米颗粒在破乳领域的应用研究进展[J]. 石油化工高等学校学报, 2021, 34(2): 9-16. |
| CHEN Yiwen, WANG Rui, WEN Jie, et al. Research progress in the application of nanoparticles in the field of demulsification[J]. Journal of Petrochemical Universities, 2021, 34(2): 9-16. | |
| 69 | ZHANG Wangqing, SHI Linqi, MA Rujiang, et al. Micellization of thermo- and pH-responsive triblock copolymer of poly(ethylene glycol)-b-poly(4-vinylpyridine)-b-poly(N-isopropylacrylamide)[J]. Macromolecules, 2005, 38(21): 8850-8852. |
| 70 | 徐海燕, 任嗣利, 贾卫红, 等. 磁性可回收氟化石墨烯破乳材料的制备及乳液分离性能[J]. 高等学校化学学报, 2019, 40(3): 508-517. |
| XU Haiyan, REN Sili, JIA Weihong, et al. Preparation of magneticaly recyclable fluorinated graphene and its demulsification performance for Emulsified oily wastewater[J]. Journal of Advanced Chemistry, 2019, 40(3): 508-517. | |
| 71 | XU Haiyan, WANG Jinqing, YANG Xiaohan, et al. Magnetically recyclable graphene oxide demulsifier adapting wide pH conditions on detachment of oil in the crude oil-in-water emulsion[J]. ACS Applied Materials & Interfaces, 2021, 13(5): 6748-6757. |
| 72 | 涂福琳, 于小荣, 魏晓静, 等. Fe3O4-PEI磁化破乳剂的制备及性能研究[J]. 合成化学, 2020, 28(12): 1065-1069. |
| TU Fulin, YU Xiaorong, WEI Xiaojing, et al. Preparation and properties of Fe3O4-PEI magnetized demulsifier[J]. Chinese Journal of Synthetic Chemistry, 2020, 28(12): 1065-1069. | |
| 73 | 李哲. 磁性碳纳米材料破乳剂的合成及其性能评价[D]. 大庆: 东北石油大学, 2022. |
| LI Zhe. Synthesis and performance evaluation of magnetic carbon nanomaterial demulsifier[D]. Daqing: Northeast Petroleum University, 2022. | |
| 74 | ADEWUNMI A A, KAMAL M S, SOLLING T I. Application of magnetic nanoparticles in demulsification: A review on synthesis, performance, recyclability, and challenges[J]. Journal of Petroleum Science and Engineering, 2021, 196: 107680. |
| 75 | WANG Huanjiang, XU Haiyan, JIA Weihong, et al. Functionalized carbon black nanoparticles used for separation of emulsified oil from oily wastewater[J]. Journal of Dispersion Science and Technology, 2018, 39(4): 497-506. |
| 76 | XU Haiyan, JIA Weihong, REN Sili, et al. Stable and efficient demulsifier of functional fluorinated graphene for oil separation from emulsified oily wastewaters[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 93: 492-499. |
| 77 | CONTRERAS O S, CABANZO R, MEJÍA-OSPINO E. Crude oil/water emulsion separation using graphene oxide and amine-modified graphene oxide particles[J]. Fuel, 2019, 240: 162-168. |
| 78 | XU Yingbiao, WANG Yefei, WANG Tingyi, et al. Demulsification of heavy oil-in-water emulsion by a novel Janus graphene oxide nanosheet: Experiments and molecular dynamic simulations[J]. Molecules (Basel, Switzerland), 2022, 27(7): 2191. |
| 79 | 田宫伟, 陈颖, 王亚林, 等. 化学破乳机理及其研究进展[J]. 硅酸盐通报, 2018, 37(1): 155-159. |
| TIAN Gongwei, CHEN Ying, WANG Yalin, et al. Chemical demulsification mechanism and its research progress[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(1): 155-159. | |
| 80 | BERRY J D, NEESON M J, DAGASTINE R R, et al. Measurement of surface and interfacial tension using pendant drop tensiometry[J]. Journal of Colloid and Interface Science, 2015, 454: 226-237. |
| 81 | YE Fan, SHEN Liwei, LIU Shi, et al. Demulsification of amphiphilic gemini ionic liquids and its demulsification mechanism[J]. Chemosphere, 2022, 309: 136650. |
| 82 | DELGADO-LINARES J G, ALVARADO J G, VÉJAR F, et al. Breaking of water-in-crude oil emulsions. 7. demulsifier performance at optimum formulation for various extended surfactant structures[J]. Energy & Fuels, 2016, 30(9): 7065-7073. |
| 83 | ABULLAH M M, AL-LOHEDAN H A, ATTAH A M. Synthesis and application of amphiphilic ionic liquid based on acrylate copolymers as demulsifier and oil spill dispersant[J]. Journal of Molecular Liquids, 2016, 219: 54-62. |
| 84 | JIANG Xia, YE Fan, ZHENG Lihua, et al. Multi-walled carbon nanotubes grafted by polyvinyl alcohol and its demulsification performance in oily wastewater[J]. ChemistrySelect, 2020, 5(26): 7895-7900. |
| 85 | GUPTA R K, DUNDERDALE G J, ENGLAND M W, et al. Oil/water separation techniques: A review of recent progresses and future directions[J]. Journal of Materials Chemistry A, 2017, 5(31): 16025-16058. |
| 86 | SUN Taolei, ZHANG Lu, WANG Yiyang, et al. Influence of demulsifiers of different structures on interfacial dilational properties of an oil-water interface containing surface-active fractions from crude oil[J]. Journal of Colloid and Interface Science, 2002, 255(2): 241-247. |
| 87 | ZHANG Lifeng, YING Hao, YAN Shu, et al. Hyperbranched poly(amido amine) as an effective demulsifier for oil-in-water emulsions of microdroplets[J]. Fuel, 2018, 211: 197-205. |
| 88 | NATARAJAN Anand, XIE Jinggang, WANG Shengqun, et al. Understanding molecular interactions of asphaltenes in organic solvents using a surface force apparatus[J]. The Journal of Physical Chemistry C, 2011, 115(32): 16043-16051. |
| 89 | HUANG J, STOYANOV S R, ZENG H. A comparison study on adsorption and interaction behaviors of diluted bitumen and conventional crude oil on model mineral surface[J]. Fuel, 2019, 253: 383-391. |
| 90 | ZHANG Ling, XIE Lei, SHI Chen, et al. Mechanistic understanding of asphaltene surface interactions in aqueous media[J]. Energy & Fuels, 2017, 31(4): 3348-3357. |
| 91 | RANE J P, PAUCHARD V, COUZIS A, et al. Interfacial rheology of asphaltenes at oil-water interfaces and interpretation of the equation of state[J]. Langmuir, 2013, 29(15): 4750-4759. |
| 92 | ZHANG Zhiqing, XU Guiying, WANG Fang, et al. Demulsification by amphiphilic dendrimer copolymers[J]. Journal of Colloid and Interface Science, 2005, 282(1): 1-4. |
| 93 | Philipp E, WINDHAB E J, ROK G, et al. Interfacial rheology of surface-active biopolymers: Acacia Senegal gum versus hydrophobically modifed starch[J]. Biomacromolecules, 2007, 8(11): 3458-3466. |
| 94 | GENG Xiaoheng, LI Changjun, ZHANG Lin, et al. Screening and demulsification mechanism of fluorinated demulsifier based on molecular dynamics simulation[J]. Molecules (Basel, Switzerland), 2022, 27(6): 1799. |
| 95 | 苏碧云, 冉良涛, 胡雅和, 等. 分子氧化及光电催化氧化对石油Pickering乳液的破乳研究进展[J]. 化工进展, 2021, 40(7): 3995-4002. |
| SU Biyun, RAN Liangtao, HU Yahe, et al. Research progress on demulsification of petroleum Pickering emulsion by molecular oxidation, photocatalytic oxidation and electrochemical oxidation[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3995-4002. |
| [1] | LI Songtao, ZHAO Jin, ZHANG Lifeng. Preparation of hyperbranched poly(amido amine) and its application to demulsification of oil-in-water emulsions [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 401-408. |
| [2] | ZHANG Lifeng, ZHAN Ningning, QIN Lijuan, ZHAO Xinxing, ZHOU Lishan, TENG Houkai, FANG Wenjun. Methacrylated hyperbranched polyglycerol demulsifier for O/W emulsion [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3434-3443. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||