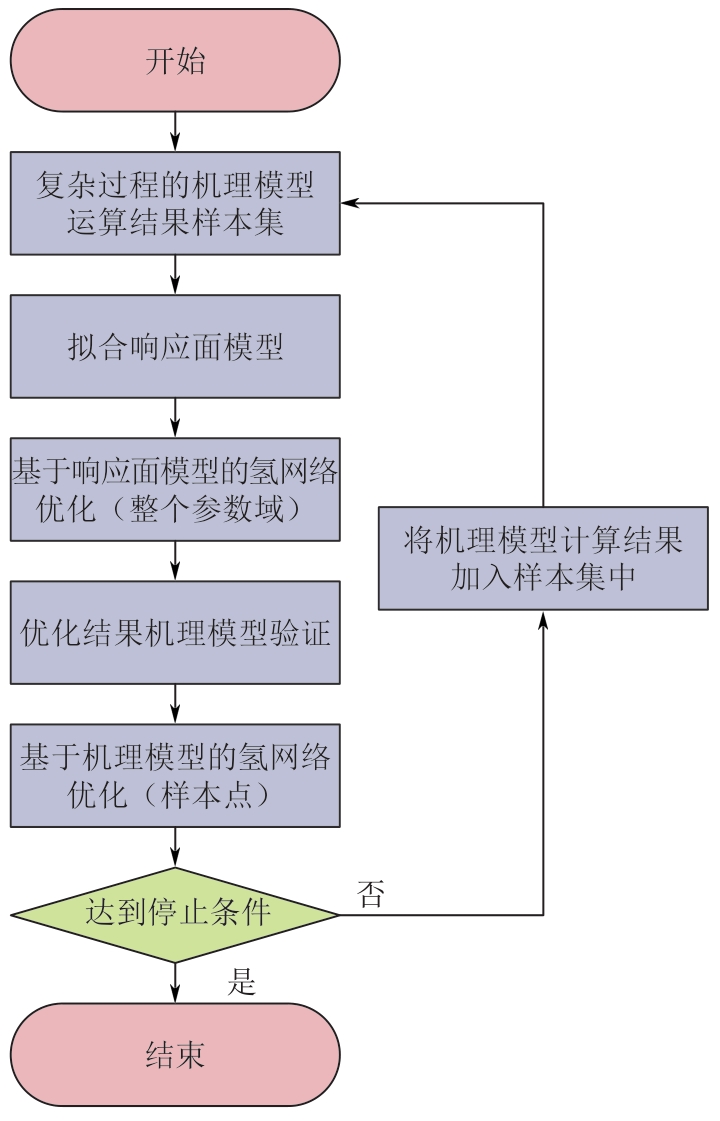
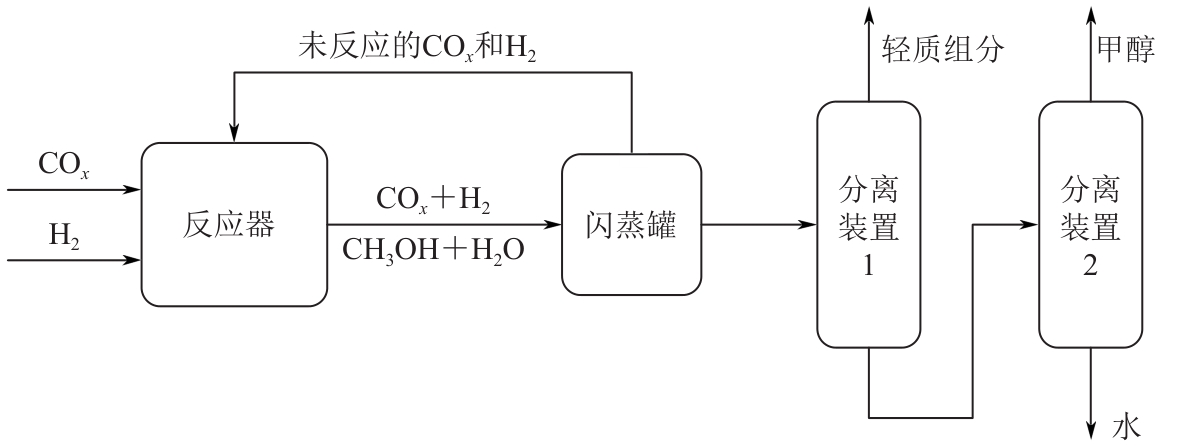
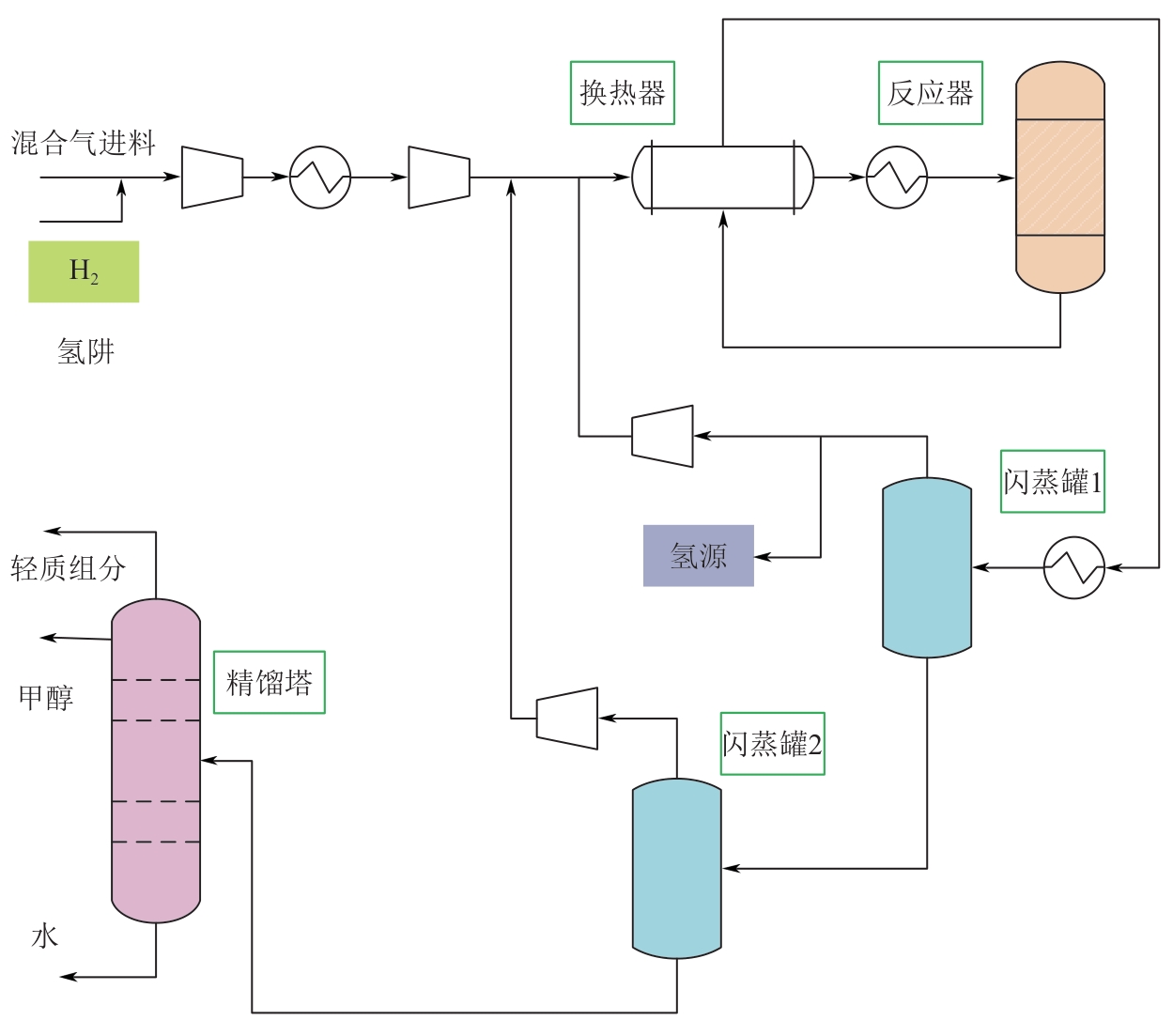
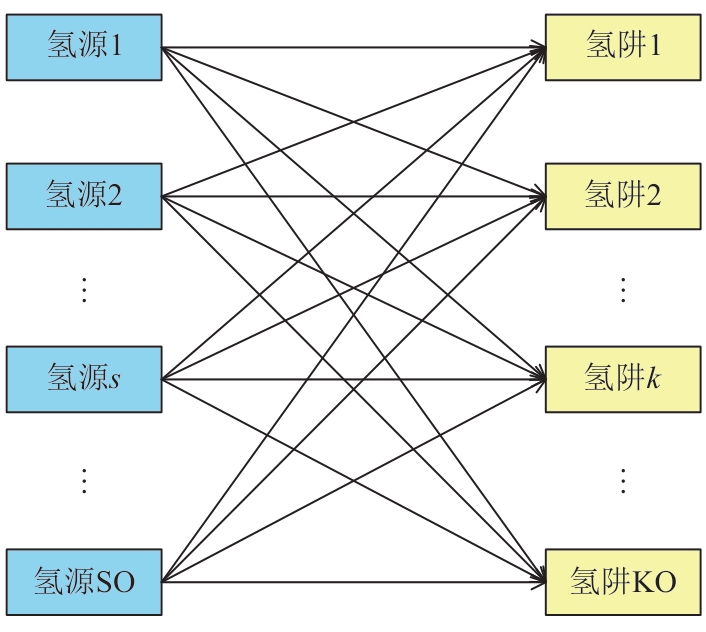
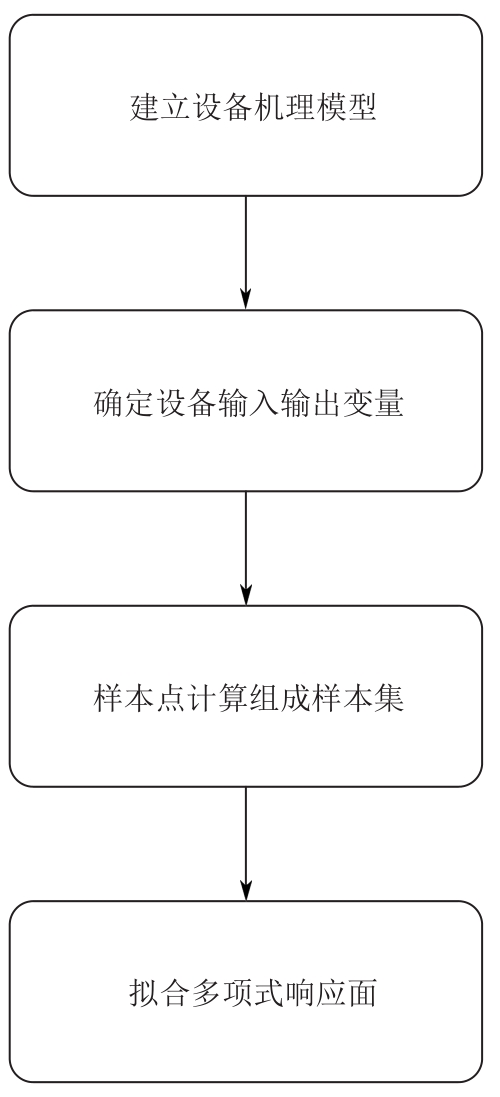
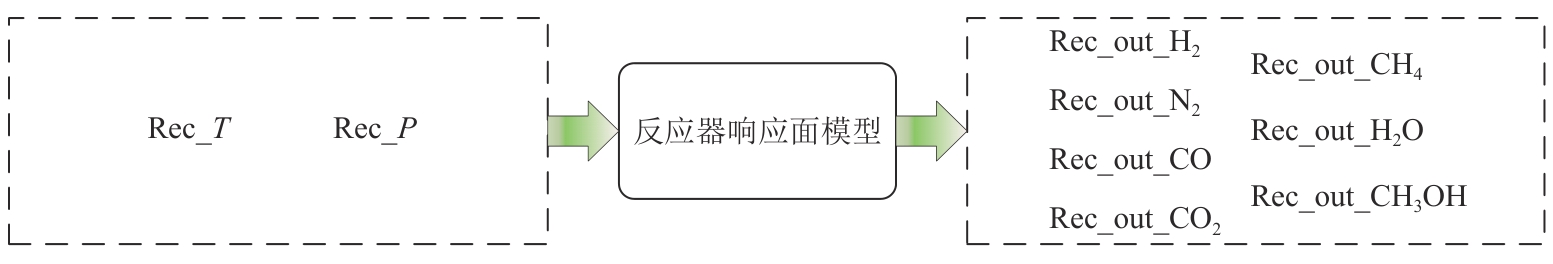
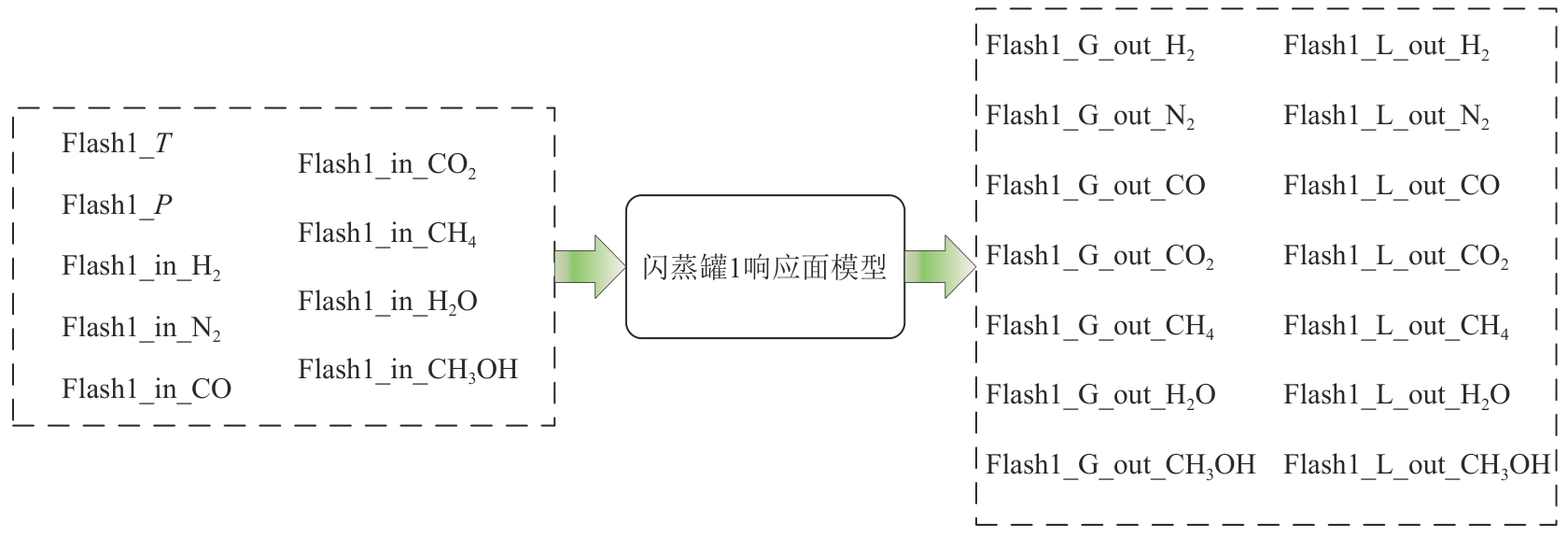
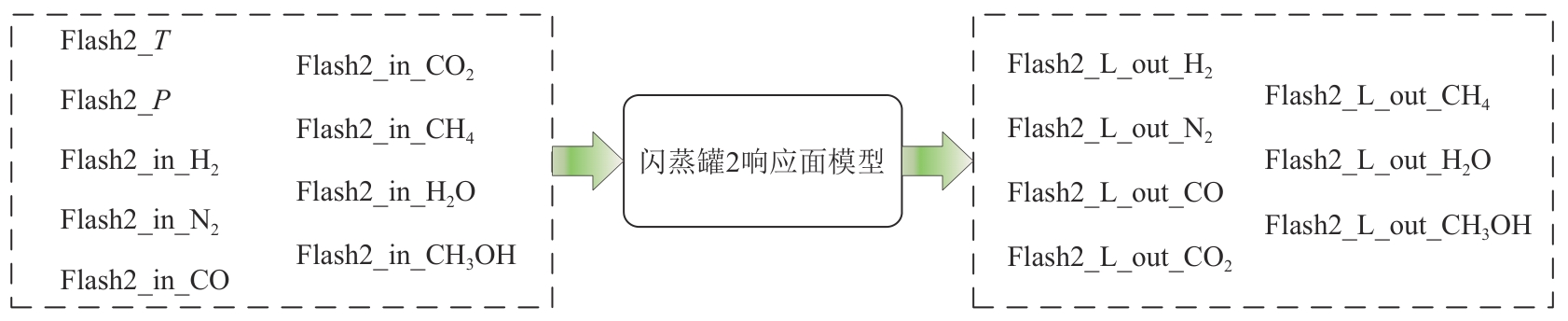
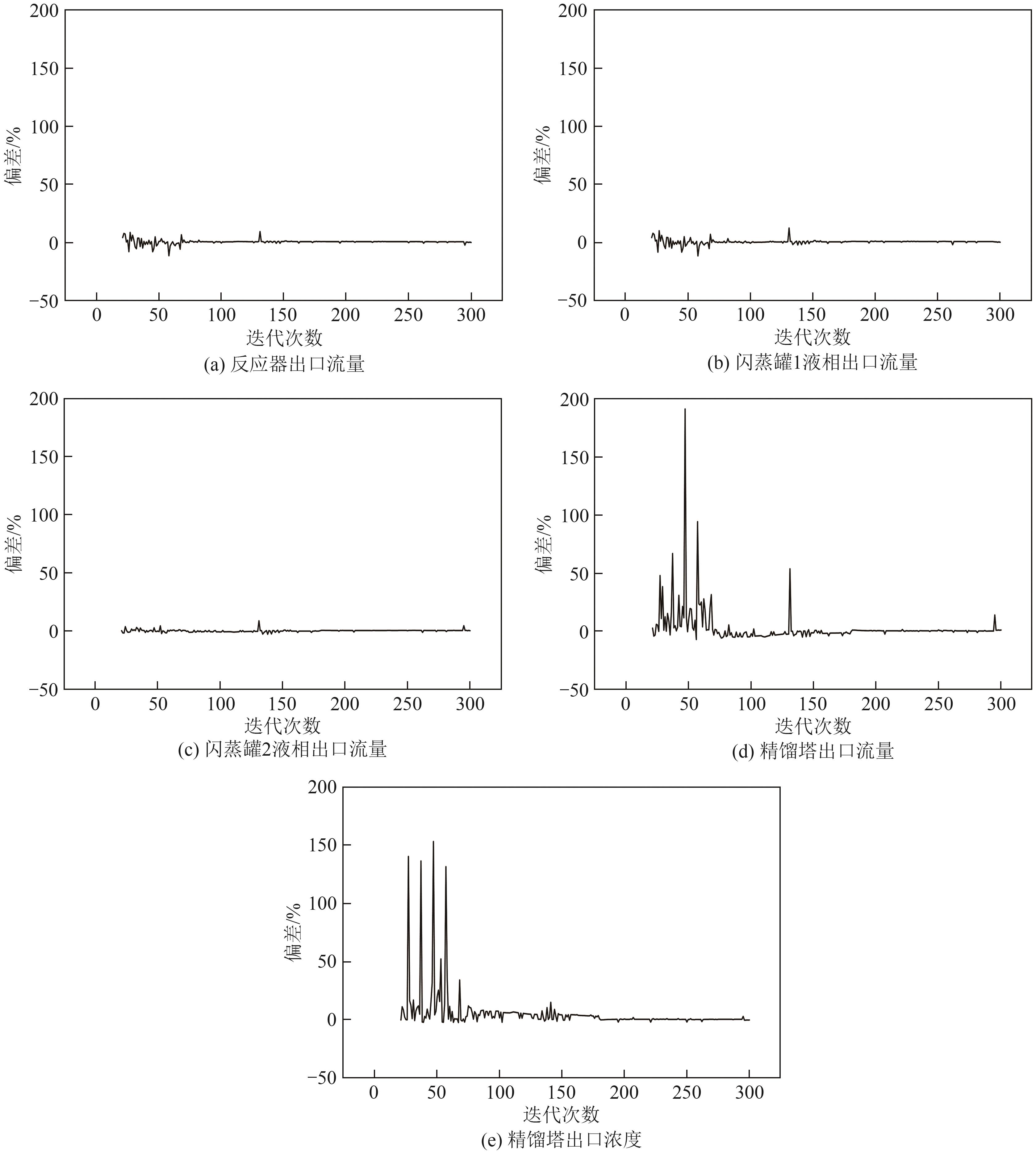
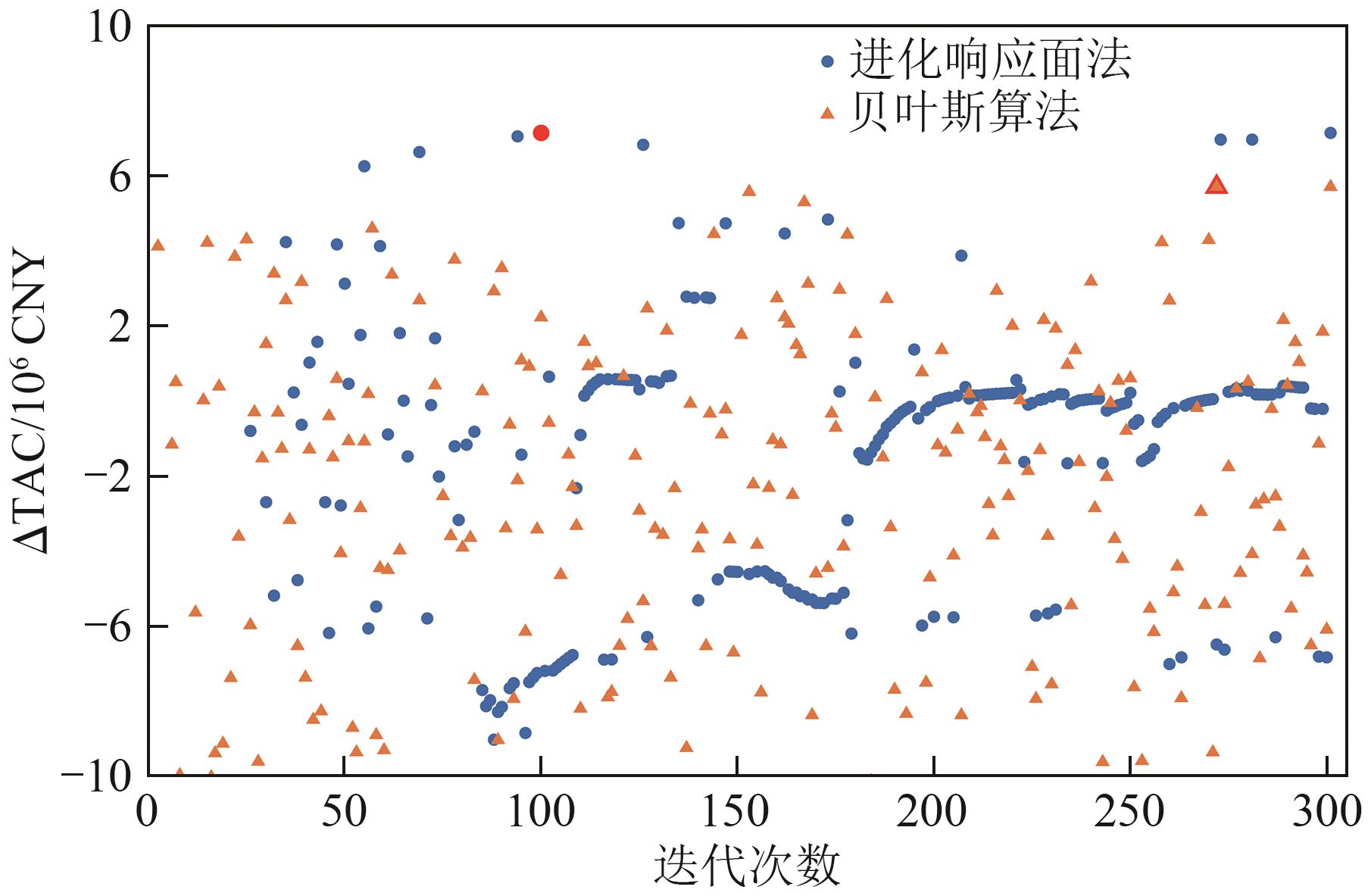
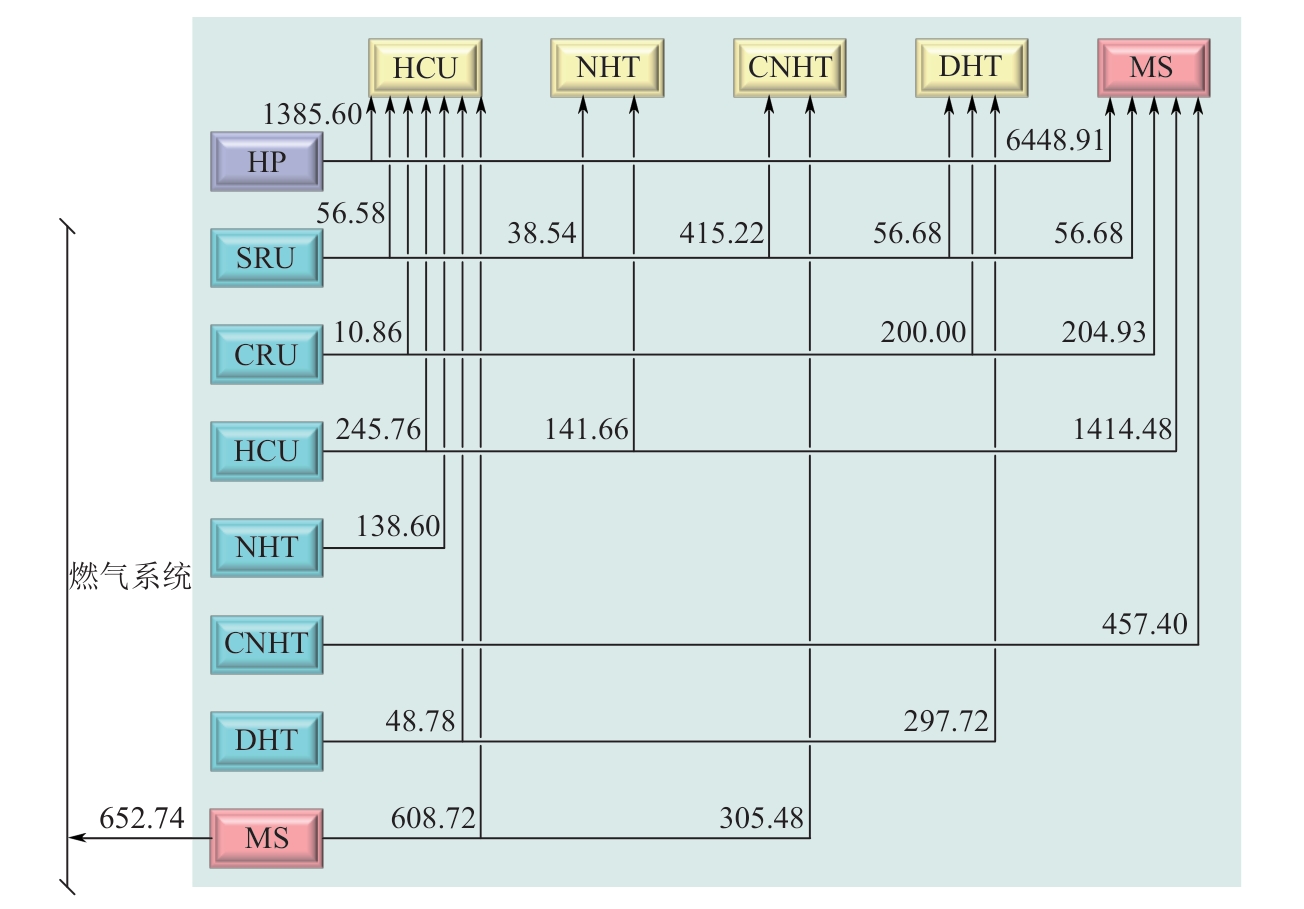
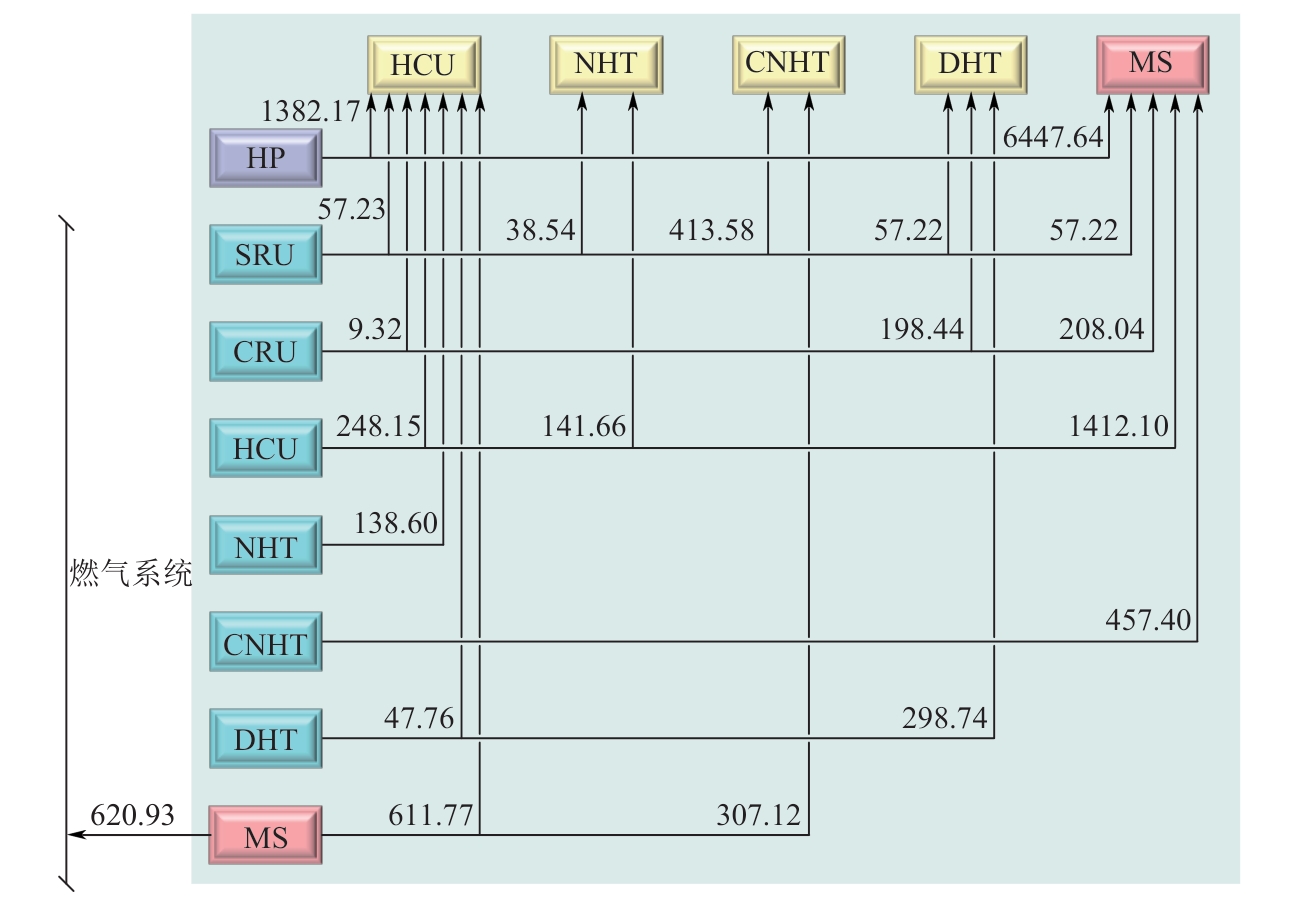
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (8): 4688-4700.DOI: 10.16085/j.issn.1000-6613.2024-1926
• Process systems modeling and simulation • Previous Articles
Simultaneous optimization of hydrogen network with CO₂ hydrogenation to methanol process based on evolutionary response surface method
HUANG Lingjun( ), ZHU Qingyu, ZHANG Yu, SUN Weiqi, DOU Dongyang(
), ZHU Qingyu, ZHANG Yu, SUN Weiqi, DOU Dongyang( ), WANG Qili(
), WANG Qili( )
)
- School of Chemical Engineering and Technology, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
-
Received:2024-11-22Revised:2024-12-24Online:2025-09-08Published:2025-08-25 -
Contact:DOU Dongyang, WANG Qili
进化响应面驱动的CO2加氢制甲醇工艺与氢网络同步优化
黄灵军( ), 朱卿宇, 张宇, 孙维祺, 窦东阳(
), 朱卿宇, 张宇, 孙维祺, 窦东阳( ), 王启立(
), 王启立( )
)
- 中国矿业大学化工学院,江苏 徐州 221116
-
通讯作者:窦东阳,王启立 -
作者简介:黄灵军(1992—),男,博士,讲师,研究方向为过程系统工程。E-mail:hlj@cumt.edu.cn。 -
基金资助:中央高校基本科研业务费专项资金项目(XJ2023003201)
CLC Number:
Cite this article
HUANG Lingjun, ZHU Qingyu, ZHANG Yu, SUN Weiqi, DOU Dongyang, WANG Qili. Simultaneous optimization of hydrogen network with CO₂ hydrogenation to methanol process based on evolutionary response surface method[J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4688-4700.
黄灵军, 朱卿宇, 张宇, 孙维祺, 窦东阳, 王启立. 进化响应面驱动的CO2加氢制甲醇工艺与氢网络同步优化[J]. 化工进展, 2025, 44(8): 4688-4700.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1926
| 反应 | M1的表达式 | M1值 | M2表达式 | M2值 |
|---|---|---|---|---|
| 式(1) | KCO | 2.16×10-5exp(46800/RT) | KCO/K1 | 9.11×107exp(-51638/RT) |
| 式(2) | 7.05×10-7exp(61700/RT) | 6.59×10-9exp(101384.87/RT) | ||
| 式(3) | 7.05×10-7exp(61700/RT) | 2.78×104exp(2946.87/RT) |
| 反应 | M1的表达式 | M1值 | M2表达式 | M2值 |
|---|---|---|---|---|
| 式(1) | KCO | 2.16×10-5exp(46800/RT) | KCO/K1 | 9.11×107exp(-51638/RT) |
| 式(2) | 7.05×10-7exp(61700/RT) | 6.59×10-9exp(101384.87/RT) | ||
| 式(3) | 7.05×10-7exp(61700/RT) | 2.78×104exp(2946.87/RT) |
| 反应 | ln M1 | ln M2 | |||
|---|---|---|---|---|---|
| A | B | A | B | ||
| 式(1) | -10.7428 | 5629.06 | 18.3272 | -6210.97 | |
| 式(2) | -14.1651 | 7421.22 | -18.8370 | 12194.48 | |
| 式(3) | -14.1651 | 7421.22 | 10.2330 | 354.45 | |
| 反应 | ln M1 | ln M2 | |||
|---|---|---|---|---|---|
| A | B | A | B | ||
| 式(1) | -10.7428 | 5629.06 | 18.3272 | -6210.97 | |
| 式(2) | -14.1651 | 7421.22 | -18.8370 | 12194.48 | |
| 式(3) | -14.1651 | 7421.22 | 10.2330 | 354.45 | |
| 项 | 常数 | A | B | |
|---|---|---|---|---|
| 1 | 1 | 0 | 0 | p |
| 2 | -18.8717 | 10103.44 | ||
| 3 | KCO | -10.7428 | 5629.06 | |
| 4 | -29.6144 | 15732.50 | ||
| 5 | -14.165 | 7421.22 | ||
| 6 | -33.037 | 17524.66 |
| 项 | 常数 | A | B | |
|---|---|---|---|---|
| 1 | 1 | 0 | 0 | p |
| 2 | -18.8717 | 10103.44 | ||
| 3 | KCO | -10.7428 | 5629.06 | |
| 4 | -29.6144 | 15732.50 | ||
| 5 | -14.165 | 7421.22 | ||
| 6 | -33.037 | 17524.66 |
| 装置 | 氢源 | 装置 | 氢阱 | ||
|---|---|---|---|---|---|
| 摩尔分数 | 流量/kmol·h-1 | 摩尔分数 | 流量/kmol·h-1 | ||
| SRU | 0.9300 | 623.80 | HCU | 0.8061 | 2495.00 |
| CRU | 0.8000 | 415.80 | NHT | 0.7885 | 180.20 |
| HCU | 0.7500 | 1801.90 | DHT | 0.7757 | 554.40 |
| NHT | 0.7500 | 138.60 | CNHT | 0.7514 | 720.70 |
| DHT | 0.7300 | 346.50 | MS | 0.9000 | 8582.41 |
| CNHT | 0.7000 | 457.40 | |||
| MS | 0.6253 | 2232.74 | |||
| HP | 0.9500 | 7782.42 | |||
| 装置 | 氢源 | 装置 | 氢阱 | ||
|---|---|---|---|---|---|
| 摩尔分数 | 流量/kmol·h-1 | 摩尔分数 | 流量/kmol·h-1 | ||
| SRU | 0.9300 | 623.80 | HCU | 0.8061 | 2495.00 |
| CRU | 0.8000 | 415.80 | NHT | 0.7885 | 180.20 |
| HCU | 0.7500 | 1801.90 | DHT | 0.7757 | 554.40 |
| NHT | 0.7500 | 138.60 | CNHT | 0.7514 | 720.70 |
| DHT | 0.7300 | 346.50 | MS | 0.9000 | 8582.41 |
| CNHT | 0.7000 | 457.40 | |||
| MS | 0.6253 | 2232.74 | |||
| HP | 0.9500 | 7782.42 | |||
| 取值 | 反应器温度/℃ | 反应器压力/bar | 闪蒸罐1温度/℃ | 闪蒸罐1压力/bar | 闪蒸罐2温度/℃ | 闪蒸罐2压力/bar |
|---|---|---|---|---|---|---|
| 上限 | 280 | 120 | 45 | 119 | 45 | 3 |
| 下限 | 220 | 80 | 35 | 79 | 35 | 1 |
| 取值 | 反应器温度/℃ | 反应器压力/bar | 闪蒸罐1温度/℃ | 闪蒸罐1压力/bar | 闪蒸罐2温度/℃ | 闪蒸罐2压力/bar |
|---|---|---|---|---|---|---|
| 上限 | 280 | 120 | 45 | 119 | 45 | 3 |
| 下限 | 220 | 80 | 35 | 79 | 35 | 1 |
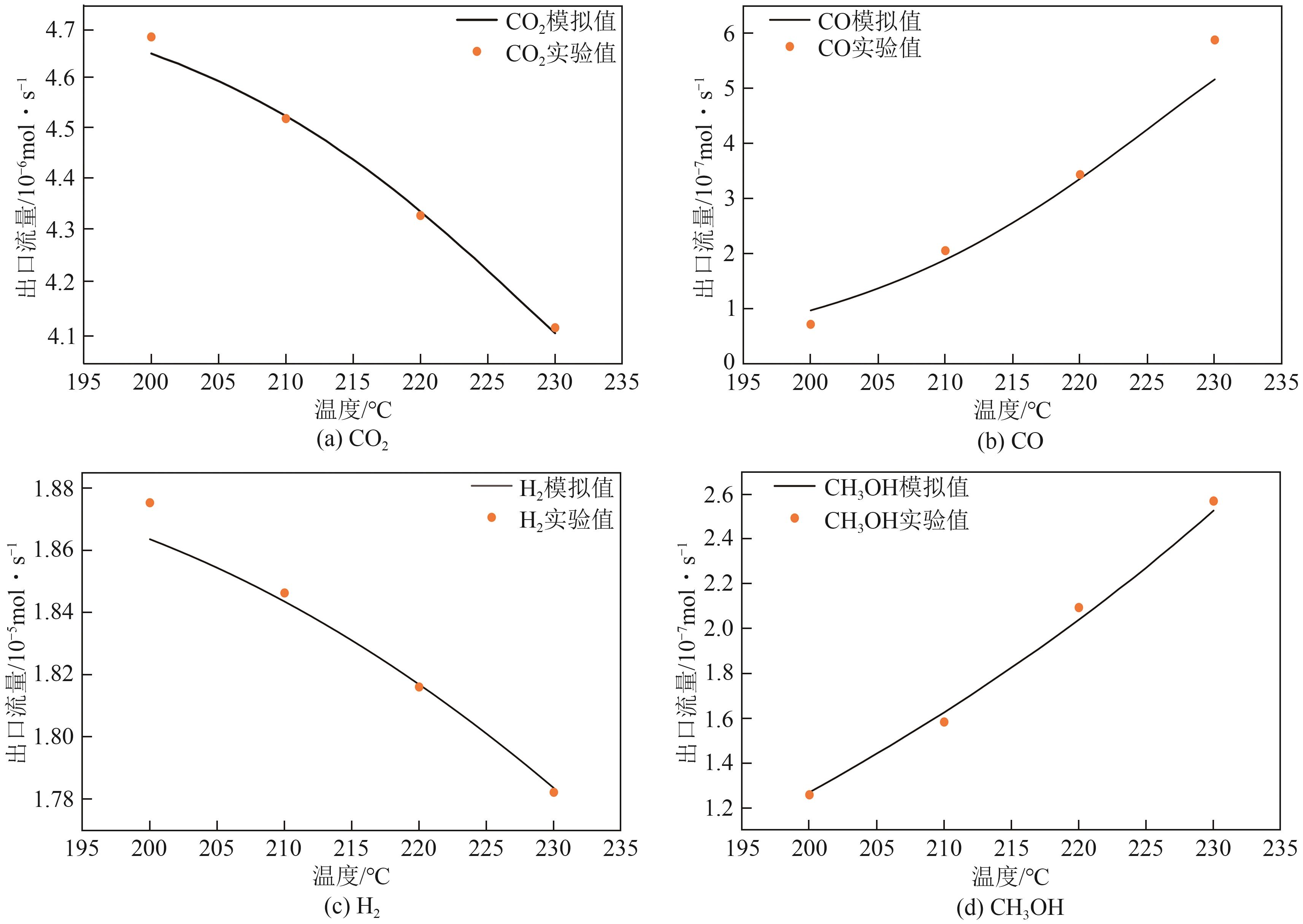
| 实验次数 | 反应体积空速(GHSV)/m3·kg-1·h-1 | P/bar | T/℃ | 组分分压/bar | 转换率(实验)/% | 选择性(实验)/% | 转换率(模拟)/% | 选择性(模拟)/% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | N2 | Ar | CO2 | H2 | CH3OH | CO | CO2 | H2 | CH3OH | CO | ||||||||
| 1 | 7800 | 50 | 200 | 34.9 | 8.9 | 6.2 | 0 | 4 .6 | 2.4 | 56 | 44 | 4.6 | 2.5 | 57 | 43 | ||||
| 2 | 7800 | 50 | 210 | 34.9 | 8.9 | 6.2 | 0 | 7.7 | 3.4 | 45 | 55 | 7.2 | 3.5 | 46 | 54 | ||||
| 3 | 7800 | 50 | 220 | 34.9 | 8.9 | 6.2 | 0 | 11.1 | 4.8 | 43 | 57 | 11.1 | 5.0 | 38 | 62 | ||||
| 4 | 7800 | 50 | 230 | 34.9 | 8.9 | 6.2 | 0 | 15.8 | 6.3 | 38 | 62 | 15.8 | 6.7 | 33 | 67 | ||||
| 5 | 7800 | 50 | 210 | 12.7 | 6.2 | 6.2 | 24.8 | 5.1 | 2.9 | 20 | 80 | 5.2 | 4.3 | 34 | 66 | ||||
| 6 | 7800 | 50 | 210 | 18.8 | 6.2 | 6.2 | 18.7 | 5.9 | 3.1 | 33 | 67 | 6.7 | 3.9 | 39 | 61 | ||||
| 7 | 7800 | 50 | 210 | 24.4 | 6.2 | 6.2 | 13.1 | 7.2 | 3.1 | 39 | 61 | 8.0 | 3.7 | 42 | 58 | ||||
| 8 | 7800 | 50 | 210 | 31.3 | 6.2 | 6.2 | 6.3 | 9.3 | 3.2 | 42 | 58 | 9.4 | 3.5 | 45 | 55 | ||||
| 9 | 7800 | 50 | 210 | 37.5 | 6.2 | 6.2 | 0 | 9.9 | 3.4 | 48 | 52 | 10.6 | 3.4 | 48 | 52 | ||||
| 10 | 7800 | 50 | 210 | 24.4 | 12 | 6.2 | 7.4 | 4.8 | 3.9 | 37 | 63 | 4.3 | 3.9 | 41 | 59 | ||||
| 11 | 7800 | 50 | 210 | 24.4 | 8.1 | 6.2 | 11.3 | 6.1 | 3.3 | 38 | 62 | 6.2 | 3.8 | 42 | 58 | ||||
| 12 | 7800 | 50 | 210 | 24.4 | 4.9 | 6.2 | 14.5 | 8.8 | 2.9 | 38 | 62 | 9.8 | 3.7 | 43 | 57 | ||||
| 13 | 7800 | 50 | 210 | 24.4 | 4.1 | 6.2 | 15.3 | 9.8 | 2.7 | 40 | 60 | 11.5 | 3.6 | 43 | 57 | ||||
| 14 | 7800 | 65 | 210 | 31.7 | 8.1 | 8.1 | 17.1 | 8.6 | 4.4 | 48 | 52 | 8.8 | 4.3 | 46 | 54 | ||||
| 15 | 7800 | 80 | 210 | 39 | 10 | 10 | 21 | 9.9 | 5.3 | 51 | 49 | 9.6 | 4.8 | 49 | 51 | ||||
| 16 | 11700 | 50 | 210 | 24.4 | 6.3 | 6.3 | 13.1 | 5.6 | 2.6 | 39 | 61 | 5.8 | 2.8 | 41 | 59 | ||||
| 17 | 23400 | 50 | 210 | 24.4 | 6.3 | 6.3 | 13.1 | 2.8 | 1.4 | 46 | 54 | 3.3 | 1.6 | 41 | 59 | ||||
| 实验次数 | 反应体积空速(GHSV)/m3·kg-1·h-1 | P/bar | T/℃ | 组分分压/bar | 转换率(实验)/% | 选择性(实验)/% | 转换率(模拟)/% | 选择性(模拟)/% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | N2 | Ar | CO2 | H2 | CH3OH | CO | CO2 | H2 | CH3OH | CO | ||||||||
| 1 | 7800 | 50 | 200 | 34.9 | 8.9 | 6.2 | 0 | 4 .6 | 2.4 | 56 | 44 | 4.6 | 2.5 | 57 | 43 | ||||
| 2 | 7800 | 50 | 210 | 34.9 | 8.9 | 6.2 | 0 | 7.7 | 3.4 | 45 | 55 | 7.2 | 3.5 | 46 | 54 | ||||
| 3 | 7800 | 50 | 220 | 34.9 | 8.9 | 6.2 | 0 | 11.1 | 4.8 | 43 | 57 | 11.1 | 5.0 | 38 | 62 | ||||
| 4 | 7800 | 50 | 230 | 34.9 | 8.9 | 6.2 | 0 | 15.8 | 6.3 | 38 | 62 | 15.8 | 6.7 | 33 | 67 | ||||
| 5 | 7800 | 50 | 210 | 12.7 | 6.2 | 6.2 | 24.8 | 5.1 | 2.9 | 20 | 80 | 5.2 | 4.3 | 34 | 66 | ||||
| 6 | 7800 | 50 | 210 | 18.8 | 6.2 | 6.2 | 18.7 | 5.9 | 3.1 | 33 | 67 | 6.7 | 3.9 | 39 | 61 | ||||
| 7 | 7800 | 50 | 210 | 24.4 | 6.2 | 6.2 | 13.1 | 7.2 | 3.1 | 39 | 61 | 8.0 | 3.7 | 42 | 58 | ||||
| 8 | 7800 | 50 | 210 | 31.3 | 6.2 | 6.2 | 6.3 | 9.3 | 3.2 | 42 | 58 | 9.4 | 3.5 | 45 | 55 | ||||
| 9 | 7800 | 50 | 210 | 37.5 | 6.2 | 6.2 | 0 | 9.9 | 3.4 | 48 | 52 | 10.6 | 3.4 | 48 | 52 | ||||
| 10 | 7800 | 50 | 210 | 24.4 | 12 | 6.2 | 7.4 | 4.8 | 3.9 | 37 | 63 | 4.3 | 3.9 | 41 | 59 | ||||
| 11 | 7800 | 50 | 210 | 24.4 | 8.1 | 6.2 | 11.3 | 6.1 | 3.3 | 38 | 62 | 6.2 | 3.8 | 42 | 58 | ||||
| 12 | 7800 | 50 | 210 | 24.4 | 4.9 | 6.2 | 14.5 | 8.8 | 2.9 | 38 | 62 | 9.8 | 3.7 | 43 | 57 | ||||
| 13 | 7800 | 50 | 210 | 24.4 | 4.1 | 6.2 | 15.3 | 9.8 | 2.7 | 40 | 60 | 11.5 | 3.6 | 43 | 57 | ||||
| 14 | 7800 | 65 | 210 | 31.7 | 8.1 | 8.1 | 17.1 | 8.6 | 4.4 | 48 | 52 | 8.8 | 4.3 | 46 | 54 | ||||
| 15 | 7800 | 80 | 210 | 39 | 10 | 10 | 21 | 9.9 | 5.3 | 51 | 49 | 9.6 | 4.8 | 49 | 51 | ||||
| 16 | 11700 | 50 | 210 | 24.4 | 6.3 | 6.3 | 13.1 | 5.6 | 2.6 | 39 | 61 | 5.8 | 2.8 | 41 | 59 | ||||
| 17 | 23400 | 50 | 210 | 24.4 | 6.3 | 6.3 | 13.1 | 2.8 | 1.4 | 46 | 54 | 3.3 | 1.6 | 41 | 59 | ||||
| 项目 | 贝叶斯算法 | 进化响应面法 |
|---|---|---|
| 反应器温度/℃ | 247.19 | 237.9 |
| 反应器压力/bar | 112.42 | 120 |
| 闪蒸罐1温度/℃ | 35 | 35 |
| 闪蒸罐1压力/bar | 111.42 | 119 |
| 闪蒸罐2温度/℃ | 39.9 | 35 |
| 闪蒸罐2压力/bar | 1.76 | 1.4 |
| 氢气公用工程用量/kmol·h-1 | 7829.81 | 8034.51 |
| 甲醇产量/kmol·h-1 | 3291.56 | 3295.32 |
| 闪蒸罐1材料成本/CNY | 482114.57 | 494919.77 |
| 年度总效益增量/CNY | 5722022.60 | 7163708.71 |
| 最优点迭代次数 | 272 | 100 |
| 计算时间/s | 5333 | 4507 |
| 项目 | 贝叶斯算法 | 进化响应面法 |
|---|---|---|
| 反应器温度/℃ | 247.19 | 237.9 |
| 反应器压力/bar | 112.42 | 120 |
| 闪蒸罐1温度/℃ | 35 | 35 |
| 闪蒸罐1压力/bar | 111.42 | 119 |
| 闪蒸罐2温度/℃ | 39.9 | 35 |
| 闪蒸罐2压力/bar | 1.76 | 1.4 |
| 氢气公用工程用量/kmol·h-1 | 7829.81 | 8034.51 |
| 甲醇产量/kmol·h-1 | 3291.56 | 3295.32 |
| 闪蒸罐1材料成本/CNY | 482114.57 | 494919.77 |
| 年度总效益增量/CNY | 5722022.60 | 7163708.71 |
| 最优点迭代次数 | 272 | 100 |
| 计算时间/s | 5333 | 4507 |
| [1] | International Energy Agency. Global hydrogen review 2024[R/OL]. (2024-10-02)[2024-11-22].. |
| [2] | International Energy Agency. CO2 emissions in 2022[R/OL]. (2023-03-02)[2024-11-22].. |
| [3] | 杨学萍. 碳中和背景下现代煤化工技术路径探索[J].化工进展, 2022, 41(7): 3402-3412. |
| YANG Xueping. Exploration on technical path of modern coal chemical industry under the background of carbon neutralization[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3402-3412. | |
| [4] | 王江涛, 鹿晓斌. CO2促进“甲醇经济”与“氢经济”共同发展[J]. 现代化工, 2021, 41(7): 14-18, 25. |
| WANG Jiangtao, LU Xiaobin. Together development of “methanol economy” and “hydrogen economy” driven by CO2 utilization[J]. Modern Chemical Industry, 2021, 41(7): 14-18, 25. | |
| [5] | AN Xin, ZUO Yizan, ZHANG Qiang, et al. Methanol synthesis from CO2 hydrogenation with a Cu/Zn/Al/Zr fibrous catalyst[J]. Chinese Journal of Chemical Engineering, 2009, 17(1): 88-94. |
| [6] | PORTHA Jean-François, PARKHOMENKO Ksenia, KOBL Kilian, et al. Kinetics of methanol synthesis from carbon dioxide hydrogenation over copper-zinc oxide catalysts[J]. Industrial & Engineering Chemistry Research, 2017, 56(45): 13133-13145. |
| [7] | KISS Anton A, PRAGT J J, VOS H J, et al. Novel efficient process for methanol synthesis by CO2 hydrogenation[J]. Chemical Engineering Journal, 2016, 284: 260-269. |
| [8] | YANG Minbo, ZENG Siying, FENG Xiao, et al. Simulation-based modeling and optimization for refinery hydrogen network integration with light hydrocarbon recovery[J]. International Journal of Hydrogen Energy, 2022, 47(7): 4662-4673. |
| [9] | HUANG Lingjun, HONG Xiaodong, LIAO Zuwei, et al. Efficient hybrid strategy for simultaneous design of refinery hydrogen networks and pressure swing adsorption unit[J]. Journal of Cleaner Production, 2024, 466: 142858. |
| [10] | CONN Andrew R, LE DIGABEL Sébastien. Use of quadratic models with mesh-adaptive direct search for constrained black box optimization[J]. Optimization Methods and Software, 2013, 28(1): 139-158. |
| [11] | BOUKOUVALA Fani, IERAPETRITOU Marianthi G. Surrogate-based optimization of expensive flowsheet modeling for continuous pharmaceutical manufacturing[J]. Journal of Pharmaceutical Innovation, 2013, 8(2): 131-145. |
| [12] | REGIS Rommel G. Constrained optimization by radial basis function interpolation for high-dimensional expensive black-box problems with infeasible initial points[J]. Engineering Optimization, 2014, 46(2): 218-243. |
| [13] | FORRESTER Alexander, JONES Donald. Global optimization of deceptive functions with sparse sampling[C]//12th AIAA/ISSMO Multidisciplinary Analysis and Optimization Conference. Victoria, British Columbia, Canada: AIAA, 2008. |
| [14] | REGIS Rommel G, SHOEMAKER Christine A. A quasi-multistart framework for global optimization of expensive functions using response surface models[J]. Journal of Global Optimization, 2013, 56(4): 1719-1753. |
| [15] | 吴依凡, 夏志鹏, 吉旭, 等. 基于代理模型的炼厂氢网络与脱硫系统同步优化[J]. 华东理工大学学报(自然科学版), 2023, 49(2): 176-187. |
| WU Yifan, XIA Zhipeng, JI Xu, et al. Surrogate-assisted refinery hydrogen network optimization with hydrogen sulfide removal[J]. Journal of East China University of Science and Technology, 2023, 49(2): 176-187. | |
| [16] | MARTELLI Emanuele, AMALDI Edoardo, CONSONNI Stefano. Numerical optimization of heat recovery steam cycles: Mathematical model, two-stage algorithm and applications[J]. Computers & Chemical Engineering, 2011, 35(12): 2799-2823. |
| [17] | ABRAMSON Mark A, AUDET Charles. Convergence of mesh adaptive direct search to second-order stationary points[J]. SIAM Journal on Optimization, 2006, 17(2): 606-619. |
| [18] | GRAAF G H, SCHOLTENS H, STAMHUIS E J, et al. Intra-particle diffusion limitations in low-pressure methanol synthesis[J]. Chemical Engineering Science, 1990, 45(4): 773-783. |
| [19] | GRAAF G H, SIJTSEMA P J J M, STAMHUIS E J, et al. Chemical equilibria in methanol synthesis[J]. Chemical Engineering Science, 1986, 41(11): 2883-2890. |
| [20] | LUYBEN William L. Design and control of a methanol reactor/column process[J]. Industrial & Engineering Chemistry Research, 2010, 49(13): 6150-6163. |
| [21] | ALVES Joao J, TOWLER Gavin P. Analysis of refinery hydrogen distribution systems[J]. Industrial & Engineering Chemistry Research, 2002, 41(23): 5759-5769. |
| [1] | WU Bo, MA Linxuan, ZHANG Mingfeng, CAO Lijuan, ZHOU Lei, WANG Xuezhong. Prediction of hydrotalcite particle size distribution based on machine learning ultrasonic attenuation [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4365-4374. |
| [2] | ZHAO Yongming, BU Yifeng, WANG Tao, DU Bing, MEN Zhuowu. Integrated optimization of catalyst dynamic replacement and steady-state Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4536-4544. |
| [3] | YANG Ao, DENG Wei, LI Yong, LUO Jing, WANG Zilin, ZHANG Jun, SHEN Weifeng. Multi-objective optimization design of triple-column pressure-swing distillation for separating ternary azeotropic mixture tetrahydrofuran/methanol/ethanol by thermodynamic topology theory [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4582-4593. |
| [4] | DONG Fenglian, LI Peng, WEI Zhiwei, SUN Xin, XU Hekai, HE Chang. Optimization of mixing processing considering crude oil procurement selection [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4648-4656. |
| [5] | YANG Jiacong, CHENG Guangxu, JIA Tonghua, JIANG Zhao. Simulation and techno-economic analysis of new efficient coupling processes between coal to methanol and green hydrogen [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4657-4668. |
| [6] | HUANG Xukun, GE Jijun, XU Pan, BI Rongshan, LI Guoxuan. Simulation and optimization of polyarylester multi-stage countercurrent washing process [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4680-4687. |
| [7] | YU Ning, WANG Qiuyue, WANG Zhicai, GAO Ziyi, CHAI Yongming, DONG Bin. Double-sites synergistic regulation for boosting water oxidation of La1-x Ni1-y Fe y O3‑δ [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3976-3984. |
| [8] | YE Xiaosheng, YUAN Ting, JIA Xin, REN Qingxia. Research progress on the removal of microcystin-LR by multicomponent composite nanomaterials [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4144-4157. |
| [9] | ZHENG Huizhe, WANG Haoze, JIANG Jie, ZHAO Ling, XI Zhenhao. Modeling and simulation of PCTG copolymer rotating disc reactor based on the coupling of reaction and mass transfer [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3372-3381. |
| [10] | SHAN Linghai, DUAN Huanhuan, ZHENG Xuming, HUANG Xiaohuang, CUI Guomin. A new competitive enhancement strategy for heat exchange units and optimization of heat exchange networks [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3393-3404. |
| [11] | LI Hongwei, XU Hanqiao, ZHAO Yan, LIU Yaozong, TENG Zhijun, JI Dong, LI Guixian. Research progress and prospect of platinum-based catalysts for electrocatalytic methanol oxidation [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3443-3456. |
| [12] | LI Ming, ZHOU Yi, NAN Lan, YE Xiaosheng. Advances in automatic optimization of continuous synthesis [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3190-3198. |
| [13] | ZHOU Penghui, ZENG Lin, DAI Li, FENG Xiaobo, NI Di. Numerical calculation of multi-objective performance optimization of a centrifugal fan based on response surface methodology and entropy weighting method [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3271-3279. |
| [14] | WU Mengqin, WANG Jiayao, XU Youqiang, WANG Yu. Progress in cascade conversion of CO2 to single cell protein through chemical and biological catalysis [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2429-2440. |
| [15] | WANG Yuanyuan, ZHANG Chong, HAN Shuangyan, XING Xinhui. Research progress on bioproduction of recombinant proteins by Pichia pastoris utilizing methanol [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2441-2450. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||