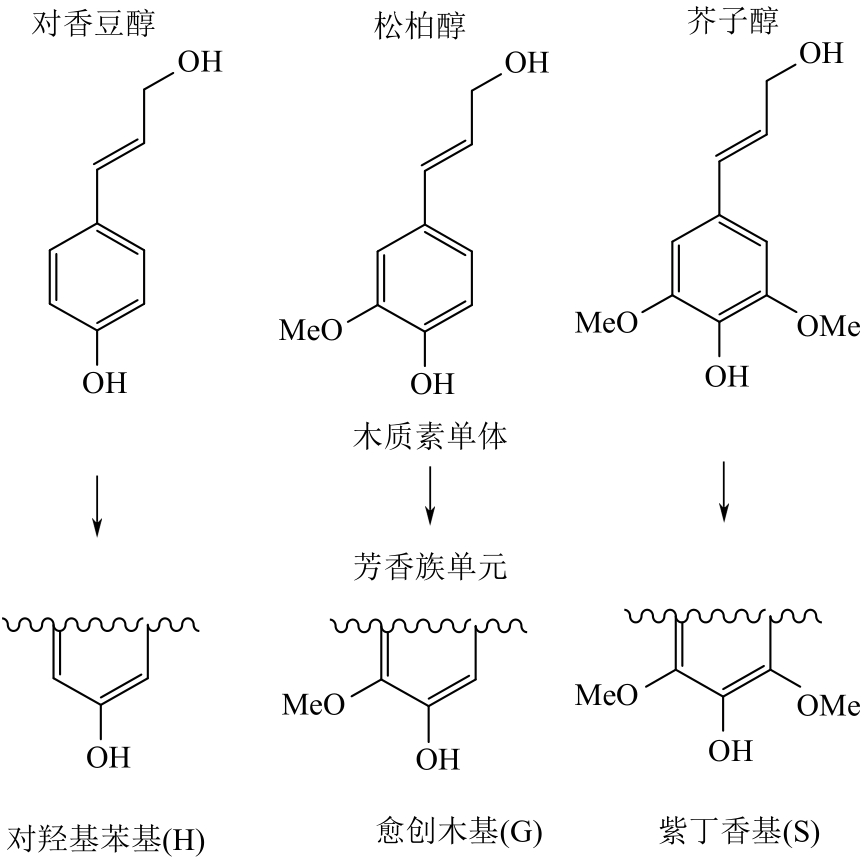
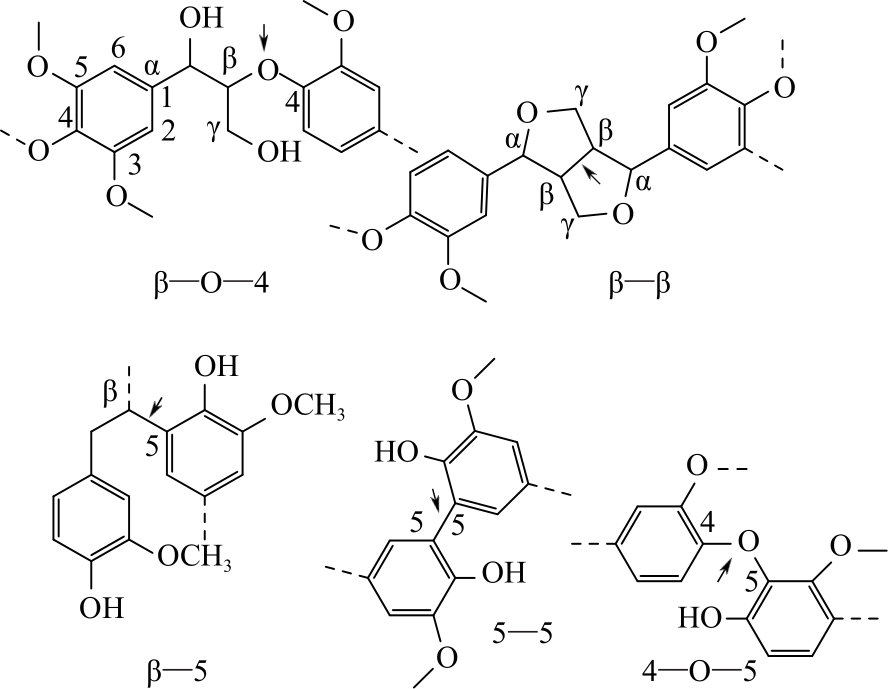
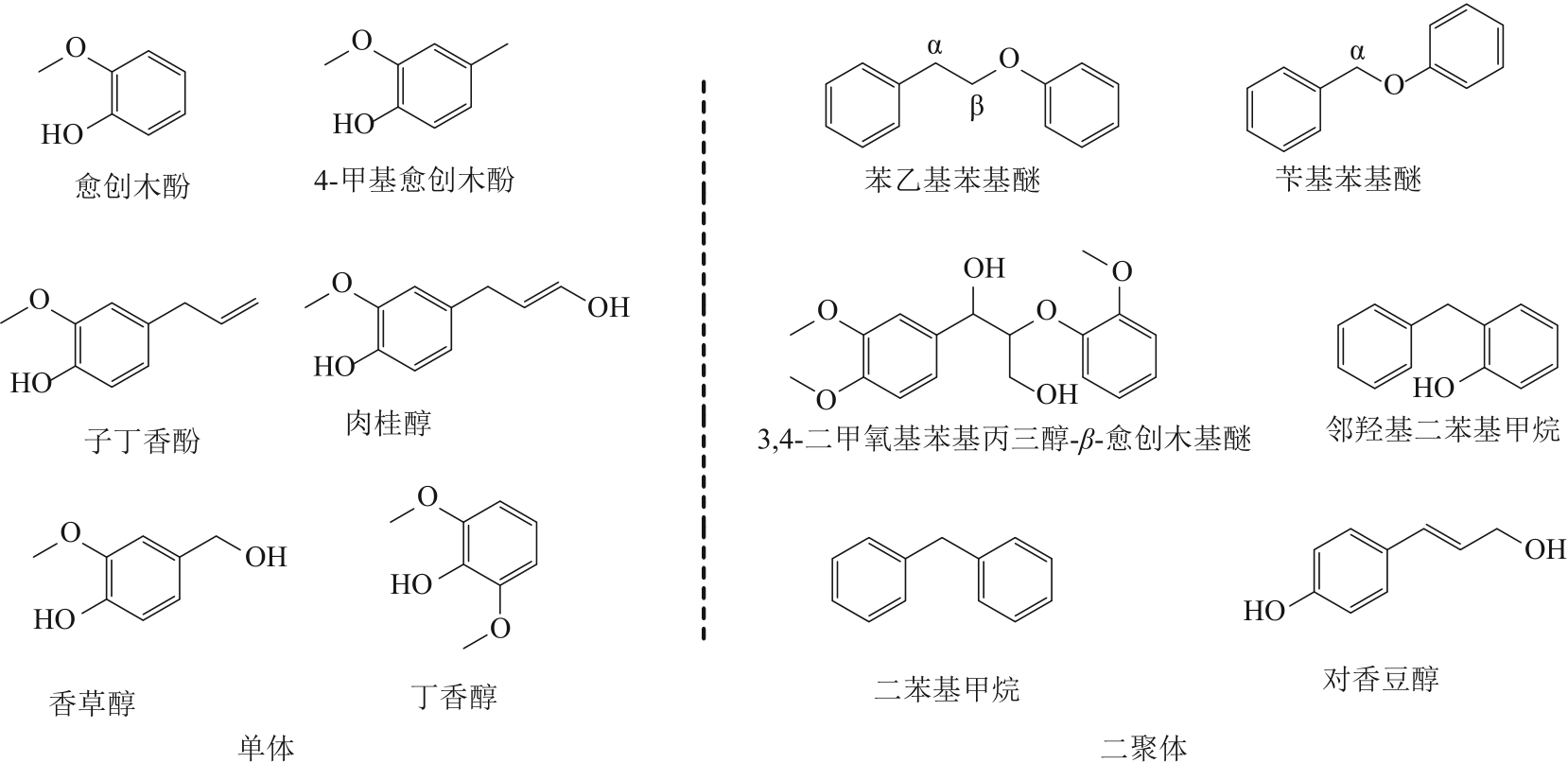
| 1 |
WENG Caihong, PENG Xiaowei, HAN Yejun. Depolymerization and conversion of lignin to value-added bioproducts by microbial and enzymatic catalysis[J]. Biotechnology for Biofuels, 2021, 14(1): 84.
|
| 2 |
BAGHEL Swati, ANANDKUMAR J. Biodepolymerization of Kraft lignin for production and optimization of vanillin using mixed bacterial culture[J]. Bioresource Technology Reports, 2019, 8: 100335.
|
| 3 |
沈晓骏, 黄攀丽, 文甲龙, 等. 木质素氧化还原解聚研究现状[J]. 化学进展, 2017, 29(1): 162-178.
|
|
SHEN Xiaojun, HUANG Panli, WEN Jialong, et al. Research status of lignin oxidative and reductive depolymerization[J]. Progress in Chemistry, 2017, 29(1): 162-178.
|
| 4 |
LIU Wujun, JIANG Hong, YU Hanqing. Thermochemical conversion of lignin to functional materials: A review and future directions[J]. Green Chemistry, 2015, 17(11): 4888-4907.
|
| 5 |
LENG Erwei, GUO Yilin, CHEN Jingwei, et al. A comprehensive review on lignin pyrolysis: Mechanism, modeling and the effects of inherent metals in biomass[J]. Fuel, 2022, 309: 122102.
|
| 6 |
张雷, 王海英, 韩洪晶, 等. 木质素催化热解用催化剂的研究进展[J]. 化工进展, 2022, 41(5):2429-2440.
|
|
ZHANG Lei, WANG Haiying, HAN Hongjing, et al. Development of catalysts for catalytic pyrolysis of lignin[J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2429-2440.
|
| 7 |
LIU Zhihua, LE Rosemary K, KOSA Matyas, et al. Identifying and creating pathways to improve biological lignin valorization[J]. Renewable and Sustainable Energy Reviews, 2019, 105: 349-362.
|
| 8 |
MA Hongwei, LI Haowei, ZHAO Weijie, et al. Selective depolymerization of lignin catalyzed by nickel supported on zirconium phosphate[J]. Green Chemistry, 2019, 21(3): 658-668.
|
| 9 |
LIU Chao, WU Shiliang, ZHANG Huiyan, et al. Catalytic oxidation of lignin to valuable biomass-based platform chemicals: A review[J]. Fuel Processing Technology, 2019, 191: 181-201.
|
| 10 |
CIRIMINNA Rosaria, FIDALGO Alexandra, MENEGUZZO Francesco, et al. Vanillin: The case for greener production driven by sustainability megatrend[J]. ChemistryOpen, 2019, 8(6): 660-667.
|
| 11 |
BANERJEE Goutam, CHATTOPADHYAY Pritam. Vanillin biotechnology: The perspectives and future[J]. Journal of the Science of Food and Agriculture, 2019, 99(2): 499-506.
|
| 12 |
TARABANKO V E, CHELBINA Yu V, KUDRYASHEV A V, et al. Separation of vanillin and syringaldehyde produced from lignins[J]. Separation Science and Technology, 2013, 48(1): 127-132.
|
| 13 |
彭建军, 张学铭, 陈雪梅, 等. 基于生物质精炼的木质素分离及结构研究进展[C]//中国造纸学会第十九届学术年会论文集. 郑州, 2020: 339-347.
|
|
WU Miao, PENG Jianjun, ZHANG Xueming, et al. Research progress on lignin separation and structure based on biomass refining[C]//Proceedings of the 19th academic annual meeting of the china paper industry association. Zhengzhou, 2020: 339-347.
|
| 14 |
LI Changzhi, ZHAO Xiaochen, WANG Aiqin, et al. Catalytic transformation of lignin for the production of chemicals and fuels[J]. Chemical Reviews, 2015, 115(21): 11559-11624.
|
| 15 |
ZHANG Chaofeng, WANG Feng. Catalytic lignin depolymerization to aromatic chemicals[J]. Accounts of Chemical Research, 2020, 53(2): 470-484.
|
| 16 |
SUN Zhuohua, Bálint FRIDRICH, DE SANTI Alessandra, et al. Bright side of lignin depolymerization: Toward new platform chemicals[J]. Chemical Reviews, 2018, 118(2): 614-678.
|
| 17 |
邱学青, 楼宏铭, 杨东杰, 等. 工业木质素的改性及其作为精细化工产品的研究进展[J]. 精细化工, 2005, 22(3): 161-167, 197.
|
|
QIU Xueqing, LOU Hongming, YANG Dongjie, et al. Research progress of industrial lignin modification and its utilization as fine chemicals[J]. Fine Chemicals, 2005, 22(3): 161-167, 197.
|
| 18 |
BOARINO Alice, KLOK Harm-Anton. Opportunities and challenges for lignin valorization in food packaging, antimicrobial, and agricultural applications[J]. Biomacromolecules, 2023, 24(3): 1065-1077.
|
| 19 |
赵丽莎. 木质素结构及加氢解聚对其抗氧化活性的影响[D]. 广州: 华南理工大学, 2020.
|
|
ZHAO Lisha. Effect of lignin structure and hydrogenolysis on its antioxidant activity[D]. Guangzhou: South China University of Technology, 2020.
|
| 20 |
Alexander STÜCKER, Fokko SCHÜTT, SAAKE Bodo, et al. Lignins from enzymatic hydrolysis and alkaline extraction of steam refined poplar wood: Utilization in lignin-phenol-formaldehyde resins[J]. Industrial Crops and Products, 2016, 85: 300-308.
|
| 21 |
Mikel OREGUI-BENGOECHEA, AGIRRE Ion, IRIONDO Aitziber, et al. Heterogeneous catalyzed thermochemical conversion of lignin model compounds: An overview[J]. Topics in Current Chemistry, 2019, 377(6): 1-75.
|
| 22 |
PANDEY M P, KIM C S. Lignin depolymerization and conversion: A review of thermochemical methods[J]. Chemical Engineering & Technology, 2011, 34(1): 29-41.
|
| 23 |
DEEPAK Raikwar, SAPTARSHI Majumdar, DEBAPRASAD Shee. Effects of solvents in the depolymerization of lignin into value-added products: A review[J]. Biomass Conversion and Biorefinery, 2023, 13(13): 11383-11416.
|
| 24 |
CHIO Chonlong, SAIN Mohini, QIN Wensheng. Lignin utilization: A review of lignin depolymerization from various aspects[J]. Renewable and Sustainable Energy Reviews, 2019, 107: 232-249.
|
| 25 |
KUMAR Avnish, BISWAS Bijoy, KAUR Ramandeep, et al. Hydrothermal oxidative valorisation of lignin into functional chemicals: A review[J]. Bioresource Technology, 2021, 342: 126016.
|
| 26 |
YAMAMURA Masaomi, HATTORI Takefumi, SUZUKI Shiro, et al. Microscale alkaline nitrobenzene oxidation method for high-throughput determination of lignin aromatic components[J]. Plant Biotechnology, 2010, 27(4): 305-310.
|
| 27 |
周姚红, 张晓华, 熊万明. 木质素催化氧化制备芳香醛研究进展[J]. 精细化工, 2022, 39(3): 442-453.
|
|
ZHOU Yaohong, ZHANG Xiaohua, XIONG Wanming. Research progress of preparation of aromatic aldehydes by catalytic oxidation of lignin[J]. Fine Chemicals, 2022, 39(3): 442-453.
|
| 28 |
DENG Weiping, ZHANG Hongxi, WU Xuejiao, et al. Oxidative conversion of lignin and lignin model compounds catalyzed by CeO2-supported Pd nanoparticles[J]. Green Chemistry, 2015, 17(11): 5009-5018.
|
| 29 |
IRMAK Sibel, KANG Juhyon, WILKINS Mark. Depolymerization of lignin by wet air oxidation[J]. Bioresource Technology Reports, 2020, 9: 100377.
|
| 30 |
ERDOCIA Xabier, PRADO Raquel, Javier FERNÁNDEZ-RODRÍGUEZ, et al. Depolymerization of different organosolv lignins in supercritical methanol, ethanol, and acetone to produce phenolic monomers[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 1373-1380.
|
| 31 |
马春慧, 孙晋德, 李伟, 等. 离子液体在木质素解聚领域的应用进展[J]. 林业工程学报, 2021, 6(5): 14-26.
|
|
MA Chunhui, SUN Jinde, LI Wei, et al. Application progress of ionic liquids in the field of lignin depolymerization[J]. Journal of Forestry Engineering, 2021, 6(5): 14-26.
|
| 32 |
PENG Mingming, NAKABAYASHI Manaka, KIM Kihoon, et al. Lignin depolymerization with alkaline ionic liquids and ethylene glycol in a continuous flow reactor [J]. Fuel, 2023, 335: 126960.
|
| 33 |
候其东, 鞠美庭, 李维尊, 等. 基于离子液体的生物质组分分离研究进展[J]. 化工进展,2016,35(10):3022-3031.
|
|
HOU Qidong, JU Meiting, LI Weizun,et al. Research progress on biomass fractionation using ionic liquids[J]. Chemical Industry and Engineering Progress, 2016, 35(10):3022-3031.
|
| 34 |
FRANCO Ana, DE Sudipta, BALU Alina M, et al. Selective oxidation of isoeugenol to vanillin over mechanochemically synthesized aluminosilicate supported transition metal catalysts[J]. ChemistrySelect, 2017, 2(29): 9546-9551.
|
| 35 |
ZAKZESKI Joseph, BRUIJNINCX Pieter C A, JONGERIUS Anna L, et al. The catalytic valorization of lignin for the production of renewable chemicals[J]. Chemical Reviews, 2010, 110(6): 3552-3599.
|
| 36 |
刘思洁. 过渡金属(Co, Ni, Mo)催化木质素解聚研究[D]. 广州: 华南理工大学, 2019.
|
|
LIU Sijie. Catalytic depolymerization of lignin using the transition metal(Co, Ni, Mo) catalysts[D]. Guangzhou: South China University of Technology, 2019.
|
| 37 |
SALES Fernando G, MARANHÃO Laísse C A, FILHO Nelson M Lima, et al. Experimental evaluation and continuous catalytic process for fine aldehyde production from lignin[J]. Chemical Engineering Science, 2007, 62(18/19/20): 5386-5391.
|
| 38 |
SALES Fernando G, MARANHÃO Laísse C A, LIMA FILHO Nelson M, et al. Kinetic evaluation and modeling of lignin catalytic wet oxidation to selective production of aromatic aldehydes[J]. Industrial & Engineering Chemistry Research, 2006, 45(20): 6627-6631.
|
| 39 |
安宏宇. 钙钛矿催化剂的掺杂改性及其催化热解木质素制备含氧化合物[D]. 大庆: 东北石油大学, 2018.
|
|
AN Hongyu. The doping of perovskite catalyst and its catalytic pyrolysis lignin to prepare the oxygenation[D]. Daqing: Northeast Petroleum University, 2018.
|
| 40 |
DENG Haibo, LIN Lu, SUN Yong, et al. Activity and stability of perovskite-type oxide LaCoO3 catalyst in lignin catalytic wet oxidation to aromatic aldehydes process[J]. Energy & Fuels, 2009, 23(1): 19-24.
|
| 41 |
ZHANG Junhua, DENG Haibo, LIN Lu. Wet aerobic oxidation of lignin into aromatic aldehydes catalysed by a perovskite-type oxide: LaFe(1-x)Cu(x)O3 (x=0, 0.1, 0.2)[J]. Molecules, 2009, 14(8): 2747-2757.
|
| 42 |
Ajay JHA, PATIL Kashinath R, RODE Chandrashekhar V. Mixed Co-Mn oxide-catalysed selective aerobic oxidation of vanillyl alcohol to vanillin in base-free conditions[J]. ChemPlusChem, 2013, 78(11): 1384-1392.
|
| 43 |
ZAKZESKI Joseph, Agnieszka DĘBCZAK, BRUIJNINCX Pieter C A, et al. Catalytic oxidation of aromatic oxygenates by the heterogeneous catalyst Co-ZIF-9[J]. Applied Catalysis A: General, 2011, 394(1/2): 79-85.
|
| 44 |
HERRMANN Wolfgang A, WESKAMP Thomas, ZOLLER Jochen P, et al. Methyltrioxorhenium: Oxidative cleavage of CC-double bonds and its application in a highly efficient synthesis of vanillin from biological waste[J]. Journal of Molecular Catalysis A: Chemical, 2000, 153(1/2): 49-52.
|
| 45 |
GU Xiaoli, CHENG Kanghua, MING He, et al. La-modified SBA-15/H2O2 systems for the microwave assisted oxidation of organosolv beech wood lignin[J]. Maderas Ciencia y Tecnología, 2012, 14(1): 31-41.
|
| 46 |
MATE V R, JHA A, JOSHI U D, et al. Effect of preparation parameters on characterization and activity of Co3O4 catalyst in liquid phase oxidation of lignin model substrates[J]. Applied Catalysis A: General, 2014, 487: 130-138.
|
| 47 |
何金义, 朱凯. 甲基三氧化铼催化过氧化氢氧化异丁香酚合成香兰素的研究[J]. 应用化工, 2019, 48(3): 550-553.
|
|
HE Jinyi, ZHU Kai. Synthesis of vanillin by catalytic hydrogen peroxide oxidation of iso-eugenol catalyzed by methyl-trioxide[J]. Applied Chemical Industry, 2019, 48(3): 550-553.
|
| 48 |
XU Wenbiao, LI Xiangyu, SHI Junyou. Oxidative depolymerization of cellulolytic enzyme lignin over silicotungvanadium polyoxometalates[J]. Polymers, 2019, 11(3): 564.
|
| 49 |
XIE Jinfeng, MA Guanfeng, OUYANG Xinping, et al. Metalloporphyrin as a biomimetic catalyst for the catalytic oxidative degradation of lignin to produce aromatic monomers[J]. Waste and Biomass Valorization, 2020, 11(8): 4481-4489.
|
| 50 |
FANG Zhen, MEIER Mark S. Toward the oxidative deconstruction of lignin: Oxidation of β-1 and β-5 linkages[J]. Organic & Biomolecular Chemistry, 2018, 16(13): 2330-2341.
|
| 51 |
林泽英. 杂多酸离子液体催化木质素选择性氧化研究[D]. 广州: 华南理工大学, 2020.
|
|
LIN Zeying. Selective oxidation of lignin catalyzed by polyoxometalate ionic liquids[D]. Guangzhou: South China University of Technology, 2020.
|
| 52 |
CANEVALI Carmen, ORLANDI Marco, PARDI Luca, et al. Oxidative degradation of monomeric and dimeric phenylpropanoids: Reactivity and mechanistic investigation[J]. Journal of the Chemical Society, Dalton Transactions, 2002(15): 3007-3014.
|
| 53 |
ZULETA Ernesto C, GOENAGA Gabriel A, ZAWODZINSKI Thomas A, et al. Deactivation of Co-Schiff base catalysts in the oxidation of para-substituted lignin models for the production of benzoquinones[J]. Catalysis Science & Technology, 2020, 10(2): 403-413.
|
| 54 |
KERVINEN Kaisa, KORPI Heikki, GERBRAND MESU J, et al. Mechanistic insights into the oxidation of veratryl alcohol with Co(salen) and oxygen in aqueous media: An in situ spectroscopic study[J]. European Journal of Inorganic Chemistry, 2005, 2005(13): 2591-2599.
|
| 55 |
李一鸣. 多金属氧酸盐的设计合成及在木质纤维素转化中的性能研究[D]. 长春: 东北师范大学, 2020.
|
|
LI Yiming. Design and synthesis of polyoxometalates and their activities in lignocellulose conversion[D]. Changchun: Northeast Normal University, 2020.
|
| 56 |
张俊旺. 多金属氧酸盐-离子液体氧化解聚木质素的研究[D]. 大连: 大连工业大学, 2020.
|
|
ZHANG Junwang. Study on oxidative depolymerization of lignin by polyoxometalates-ionic liquids[D]. Dalian: Dalian Polytechnic University, 2020.
|
| 57 |
KIM Yong Sik, CHANG Houmin, KADLA John F. Polyoxometalate (POM) oxidation of lignin model compounds[J]. Holzforschung, 2008, 62(1): 38-49.
|
| 58 |
VOITL Tobias, VON ROHR Philipp Rudolf. Oxidation of lignin using aqueous polyoxometalates in the presence of alcohols[J]. ChemSusChem, 2008, 1(8/9): 763-769.
|
| 59 |
DE GREGORIO Gilbert F, PRADO Raquel, VRIAMONT Charles, et al. Oxidative depolymerization of lignin using a novel polyoxometalate-protic ionic liquid system[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(11): 6031-6036.
|
| 60 |
CUI Futong, DOLPHIN David. Metallophthalocyanines as possible lignin peroxidase models[J]. Bioorganic & Medicinal Chemistry, 1995, 3(5): 471-477.
|
| 61 |
TARABANKO V E, PETUKHOV D V, SELYUTIN G E. New mechanism for the catalytic oxidation of lignin to vanillin[J]. Kinetics and Catalysis, 2004, 45(4): 569-577.
|
| 62 |
XIANG Q, LEE Y Y. Production of oxychemicals from precipitated hardwood lignin[J]. Applied Biochemistry and Biotechnology, 2001, 91/92/93: 71-80.
|
| 63 |
PARTENHEIMER W. Methodology and scope of metal/bromide autoxidation of hydrocarbons[J]. Catalysis Today, 1995, 23(2): 69-158.
|
| 64 |
马春慧, 张继芳, 李伟, 等. 电化学催化木质素解聚的研究进展[J]. 林产化学与工业, 2022, 42(1): 110-122.
|
|
MA Chunhui, ZHANG Jifang, LI Wei, et al. Research progress in electrochemically catalyzed depolymerization of lignin[J]. Chemistry and Industry of Forest Products, 2022, 42(1): 110-122.
|
| 65 |
YANG C, MALDONADO S, STEPHENSON C R J. Electrocatalytic lignin oxidation[J]. ACS Catalysis, 2021, 11(16): 10104-10114.
|
| 66 |
FANG Zhiyong, LI Fuhua, WANG Mei, et al. Selective electrocatalytic upgrading of lignin to aryl aldehydes and carboxylic acids over dodecyl sulfate-intercalated CoS nanocones[J]. Applied Catalysis B: Environmental, 2023, 323: 122149.
|
| 67 |
DU Xu, ZHANG Haichuan, SULLIVAN Kevin P, et al. Electrochemical lignin conversion[J]. ChemSusChem, 2020, 13(17): 4318-4343.
|
| 68 |
SCHMITT Dominik, REGENBRECHT Carolin, HARTMER Marius, et al. Highly selective generation of vanillin by anodic degradation of lignin: A combined approach of electrochemistry and product isolation by adsorption[J]. Beilstein Journal of Organic Chemistry, 2015, 11: 473-480.
|
| 69 |
PARPOT P, BETTENCOURT A P, CARVALHO A M, et al. Biomass conversion: Attempted electrooxidation of lignin for vanillin production[J]. Journal of Applied Electrochemistry, 2000, 30(6): 727-731.
|
| 70 |
WANG Yongsheng, YANG Fang, LIU Zhihua, et al. Electrocatalytic degradation of aspen lignin over Pb/PbO2 electrode in alkali solution[J]. Catalysis Communications, 2015, 67: 49-53.
|
| 71 |
TOLBA Rasha, TIAN Min, WEN Jiali, et al. Electrochemical oxidation of lignin at IrO2-based oxide electrodes[J]. Journal of Electroanalytical Chemistry, 2010, 649(1/2): 9-15.
|
| 72 |
许仃仃. 针叶木硫酸盐木素的电化学降解及改性研究[D]. 济南: 齐鲁工业大学, 2019.
|
|
XU Dingding. Electrochemical degradation and modification of softwood kraft lignin[D]. Jinan: Qilu University of Technology, 2019.
|
| 73 |
UĞURLU M, KARAOĞLU M H. TiO2 supported on sepiolite: Preparation, structural and thermal characterization and catalytic behaviour in photocatalytic treatment of phenol and lignin from olive mill wastewater[J]. Chemical Engineering Journal, 2011, 166(3): 859-867.
|
| 74 |
CAO Yang, CHEN Season S, ZHANG Shicheng, et al. Advances in lignin valorization towards bio-based chemicals and fuels: Lignin biorefinery[J]. Bioresource Technology, 2019, 291: 121878.
|
| 75 |
PRADO Raquel, ERDOCIA Xabier, LABIDI Jalel. Effect of the photocatalytic activity of TiO2 on lignin depolymerization[J]. Chemosphere, 2013, 91(9): 1355-1361.
|
| 76 |
GONG Jianyu, IMBAULT Alexander, FARNOOD Ramin. The promoting role of bismuth for the enhanced photocatalytic oxidation of lignin on Pt-TiO2 under solar light illumination[J]. Applied Catalysis B: Environmental, 2017, 204: 296-303.
|
| 77 |
LIU Huifang, LI Hongji, LUO Nengchao, et al. Visible-light-induced oxidative lignin C-C bond cleavage to aldehydes using vanadium catalysts[J]. ACS Catalysis, 2020, 10(1): 632-643.
|
| 78 |
NAIR Vaishakh, DHAR Piyali, VINU R. Production of phenolics via photocatalysis of ball milled lignin-TiO2 mixtures in aqueous suspension[J]. RSC Advances, 2016, 6(22): 18204-18216.
|
| 79 |
王晶, 倪金荧, 王利群, 等. 一株木质素降解细菌的筛选及其降解途径[J]. 化工进展,2021,40(7): 4021-4026.
|
|
WANG Jing, NI Jinying, WANG Liqun, et al. Screening of a lignin degrading bacterium and its degradation pathway[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 4021-4026.
|
| 80 |
SAINSBURY Paul D, MINEYEVA Yelena, MYCROFT Zoe, et al. Chemical intervention in bacterial lignin degradation pathways: Development of selective inhibitors for intradiol and extradiol catechol dioxygenases[J]. Bioorganic Chemistry, 2015, 60: 102-109.
|
| 81 |
梁丛颖, 林璐. 环境微生物介导的木质素代谢及其资源化利用研究进展[J]. 微生物学通报, 2020, 47(10): 3380-3392.
|
|
LIANG Congying, LIN Lu. Environmental microorganisms driven lignin biodegradation and their roles in lignin utilization[J]. Microbiology China, 2020, 47(10): 3380-3392.
|
| 82 |
唐亮, 廖强, 夏奡, 等. 仿生酶菌协同体系预处理木质素机理及特性[J]. 化工进展,2021,40(10): 5378-5387.
|
|
TANG Liang, LIAO Qiang, XIA Ao, et al. Mechanism and characteristics of nature inspired enzyme-fungi synergistic system for lignin pretreatment[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5378-5387.
|
| 83 |
徐杰. 光催化材料结构调控和解聚木质素研究[D]. 南京: 南京林业大学, 2021.
|
|
XU Jie. Study on the structure regulation of photocatalytic materials and the depolymerization of lignin[D]. Nanjing: Nanjing Forestry University, 2021.
|
| 84 |
Lucía PENÍN, GIGLI Matteo, SABUZI Federica, et al. Biomimetic vanadate and molybdate systems for oxidative upgrading of iono- and organosolv hard- and softwood lignins[J]. Processes, 2020, 8(9): 1161.
|
| 85 |
CRESTINI Claudia, JURASEK Lubo, ARGYROPOULOS Dimitris S. On the mechanism of the laccase-mediator system in the oxidation of lignin[J]. Chemistry—A European Journal, 2003, 9(21): 5371-5378.
|
| 86 |
BOHLIN Christina, PERSSON Per, GORTON Lo, et al. Product profiles in enzymic and non-enzymic oxidations of the lignin model compound erythro-1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)-1,3-propanediol[J]. Journal of Molecular Catalysis B: Enzymatic, 2005, 35(4/5/6): 100-107.
|
 ), BAI Yuchen1,2(
), BAI Yuchen1,2( )
)