Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (10): 5491-5498.DOI: 10.16085/j.issn.1000-6613.2020-2225
• Chemical processes and equipment • Previous Articles Next Articles
Nitration of n-hexane with NO2 and theoretical calculation
LI Xingyan1( ), WANG Mo1, DAI Xuan1, PENG Xinhua1, TANG Shuangling2(
), WANG Mo1, DAI Xuan1, PENG Xinhua1, TANG Shuangling2( )
)
- 1.School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, Jiangsu, China
2.School of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing 210094, Jiangsu, China
-
Received:2020-11-06Revised:2021-03-16Online:2021-10-25Published:2021-10-10 -
Contact:TANG Shuangling
NO2硝化正己烷及其理论计算
李星彦1( ), 王墨1, 戴璇1, 彭新华1, 唐双凌2(
), 王墨1, 戴璇1, 彭新华1, 唐双凌2( )
)
- 1.南京理工大学化学与化工学院,江苏 南京 210094
2.南京理工大学环境与生物工程学院,江苏 南京 210094
-
通讯作者:唐双凌 -
作者简介:李星彦(1996—),男,硕士研究生,研究方向为有机化学。E-mail:15061130209@163.com 。 -
基金资助:国家乏燃料后处理科研专项
CLC Number:
Cite this article
LI Xingyan, WANG Mo, DAI Xuan, PENG Xinhua, TANG Shuangling. Nitration of n-hexane with NO2 and theoretical calculation[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5491-5498.
李星彦, 王墨, 戴璇, 彭新华, 唐双凌. NO2硝化正己烷及其理论计算[J]. 化工进展, 2021, 40(10): 5491-5498.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-2225
| 序号 | 温度 /℃ | 转化率② /% | 选择性/% | ||
|---|---|---|---|---|---|
| 1-硝基己烷 | 2-硝基己烷 | 3-硝基己烷 | |||
| 1 | 100 | 29.6 | 1.2 | 58.0 | 40.9 |
| 2 | 110 | 58.9 | 1.4 | 57.6 | 41.0 |
| 3 | 120 | 64.3 | 2.3 | 56.5 | 41.2 |
| 4 | 130 | 64.8 | 1.9 | 57.2 | 40.9 |
| 5 | 140 | 65.3 | 2.1 | 56.5 | 41.4 |
| 序号 | 温度 /℃ | 转化率② /% | 选择性/% | ||
|---|---|---|---|---|---|
| 1-硝基己烷 | 2-硝基己烷 | 3-硝基己烷 | |||
| 1 | 100 | 29.6 | 1.2 | 58.0 | 40.9 |
| 2 | 110 | 58.9 | 1.4 | 57.6 | 41.0 |
| 3 | 120 | 64.3 | 2.3 | 56.5 | 41.2 |
| 4 | 130 | 64.8 | 1.9 | 57.2 | 40.9 |
| 5 | 140 | 65.3 | 2.1 | 56.5 | 41.4 |
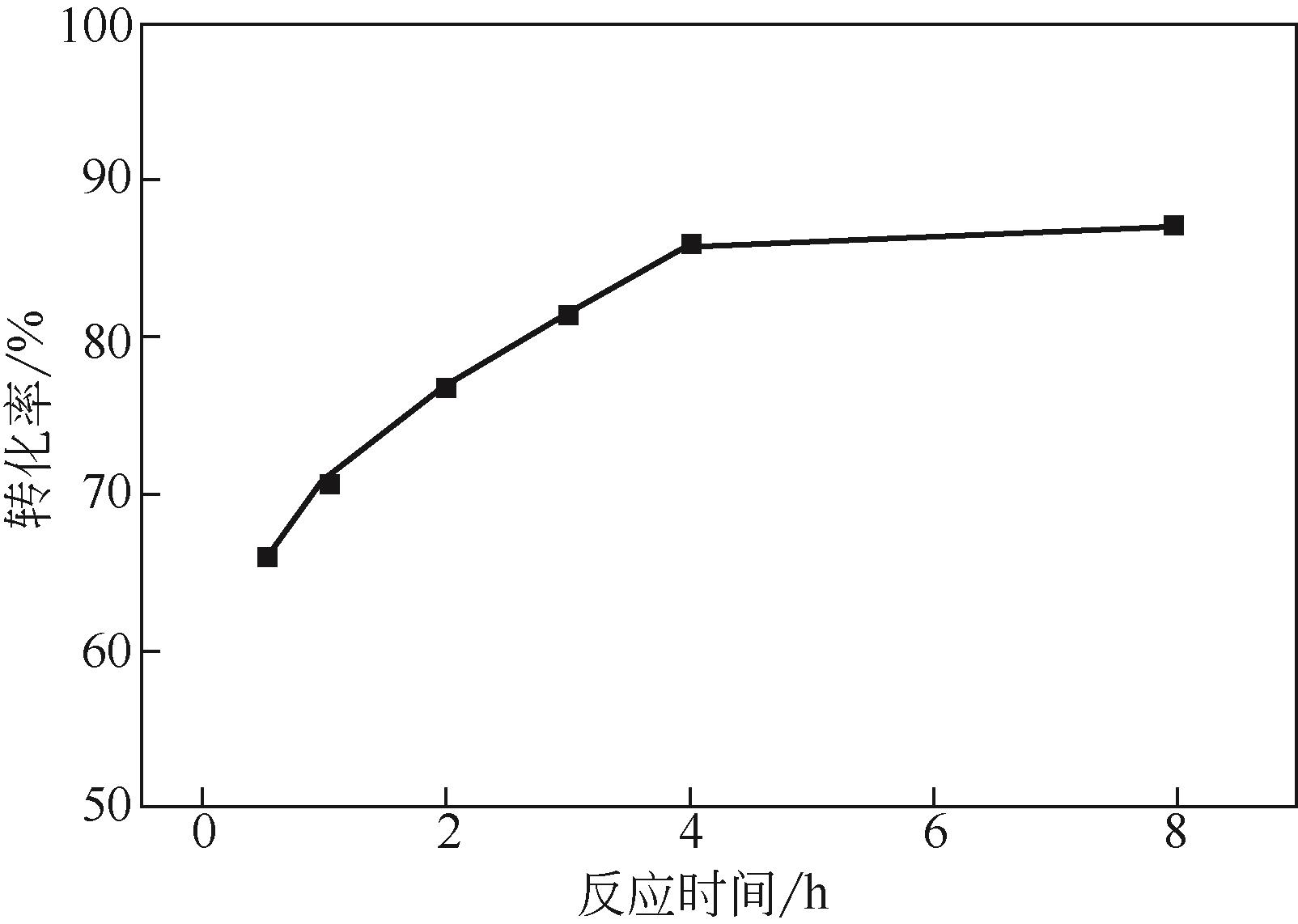
| 序号 | 正己烷∶NO2 (摩尔比) | 转化率② /% | 选择性/% | ||
|---|---|---|---|---|---|
| 1-硝基己烷 | 2-硝基己烷 | 3-硝基己烷 | |||
| 1 | 1∶0.5 | 32.0 | 1.3 | 55.9 | 42.9 |
| 2 | 1∶1 | 64.3 | 2.3 | 56.5 | 41.2 |
| 3 | 1∶1.5 | 73.0 | 2.7 | 57.0 | 41.4 |
| 4 | 1∶2 | 85.9 | 2.0 | 54.3 | 43.7 |
| 5 | 1∶2.5 | 86.1 | 1.8 | 52.2 | 46.0 |
| 序号 | 正己烷∶NO2 (摩尔比) | 转化率② /% | 选择性/% | ||
|---|---|---|---|---|---|
| 1-硝基己烷 | 2-硝基己烷 | 3-硝基己烷 | |||
| 1 | 1∶0.5 | 32.0 | 1.3 | 55.9 | 42.9 |
| 2 | 1∶1 | 64.3 | 2.3 | 56.5 | 41.2 |
| 3 | 1∶1.5 | 73.0 | 2.7 | 57.0 | 41.4 |
| 4 | 1∶2 | 85.9 | 2.0 | 54.3 | 43.7 |
| 5 | 1∶2.5 | 86.1 | 1.8 | 52.2 | 46.0 |
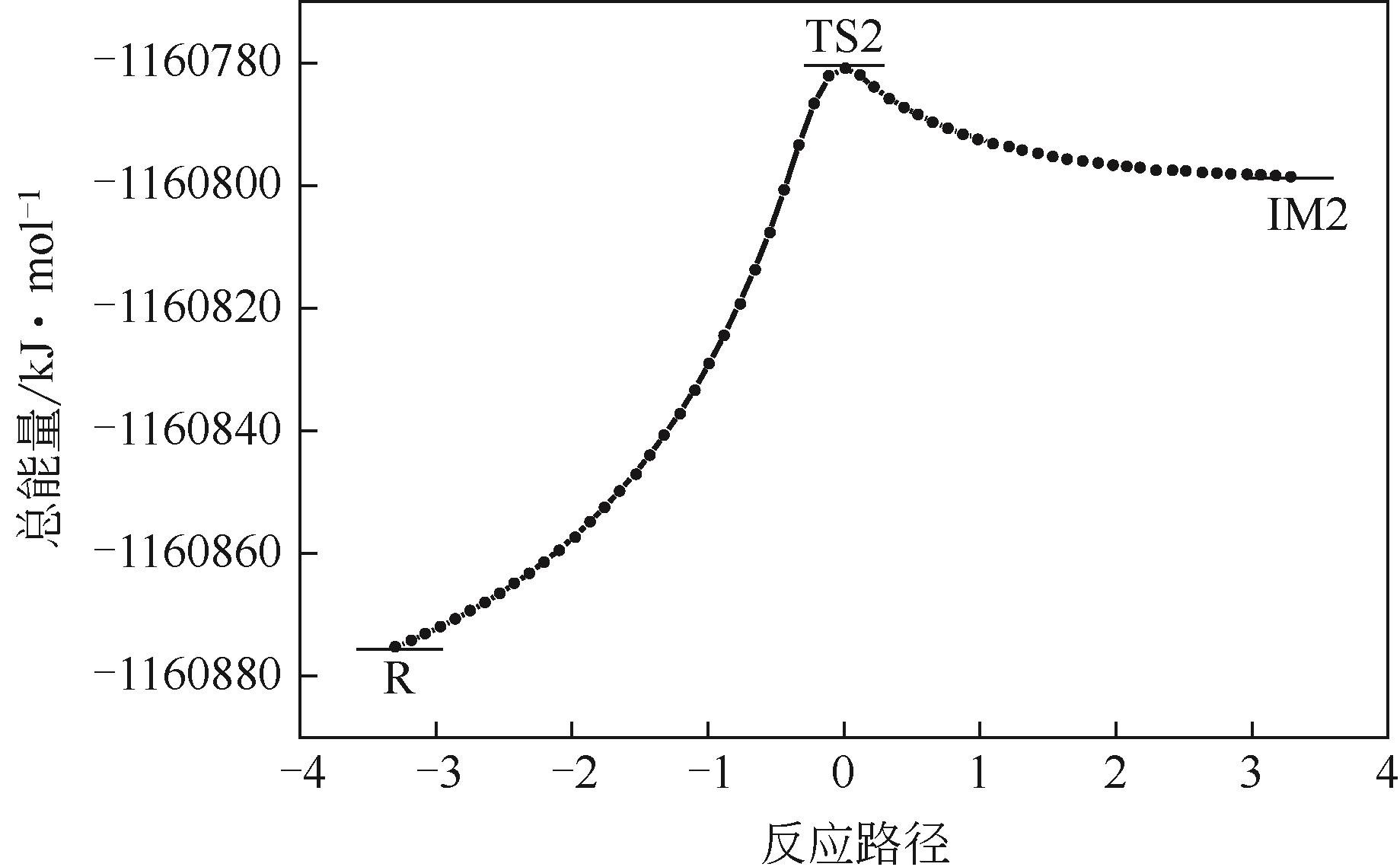
| 进攻位置 | 总能量/kJ·mol-1 | ||
|---|---|---|---|
| 途径(1) | 途径(2) | 途径(3) | |
| 1-C | |||
| ER | -1160803.565 | -1160803.155 | -1160803.708 |
| ETS | -1160694.314 | -1160701.600 | -1160538.883 |
| Ea | 109.252 | 101.556 | 264.825 |
| 2-C | |||
| ER | -1160803.595 | -1160803.618 | -1160799.290 |
| ETS | -1160710.242 | -1160715.722 | -1160524.374 |
| Ea | 93.354 | 87.896 | 274.916 |
| 3-C | |||
| ER | -1160803.507 | -1160802.418 | -1160803.395 |
| ETS | -1160708.007 | -1160713.692 | -1160532.478 |
| Ea | 95.499 | 88.727 | 270.917 |
| 进攻位置 | 总能量/kJ·mol-1 | ||
|---|---|---|---|
| 途径(1) | 途径(2) | 途径(3) | |
| 1-C | |||
| ER | -1160803.565 | -1160803.155 | -1160803.708 |
| ETS | -1160694.314 | -1160701.600 | -1160538.883 |
| Ea | 109.252 | 101.556 | 264.825 |
| 2-C | |||
| ER | -1160803.595 | -1160803.618 | -1160799.290 |
| ETS | -1160710.242 | -1160715.722 | -1160524.374 |
| Ea | 93.354 | 87.896 | 274.916 |
| 3-C | |||
| ER | -1160803.507 | -1160802.418 | -1160803.395 |
| ETS | -1160708.007 | -1160713.692 | -1160532.478 |
| Ea | 95.499 | 88.727 | 270.917 |
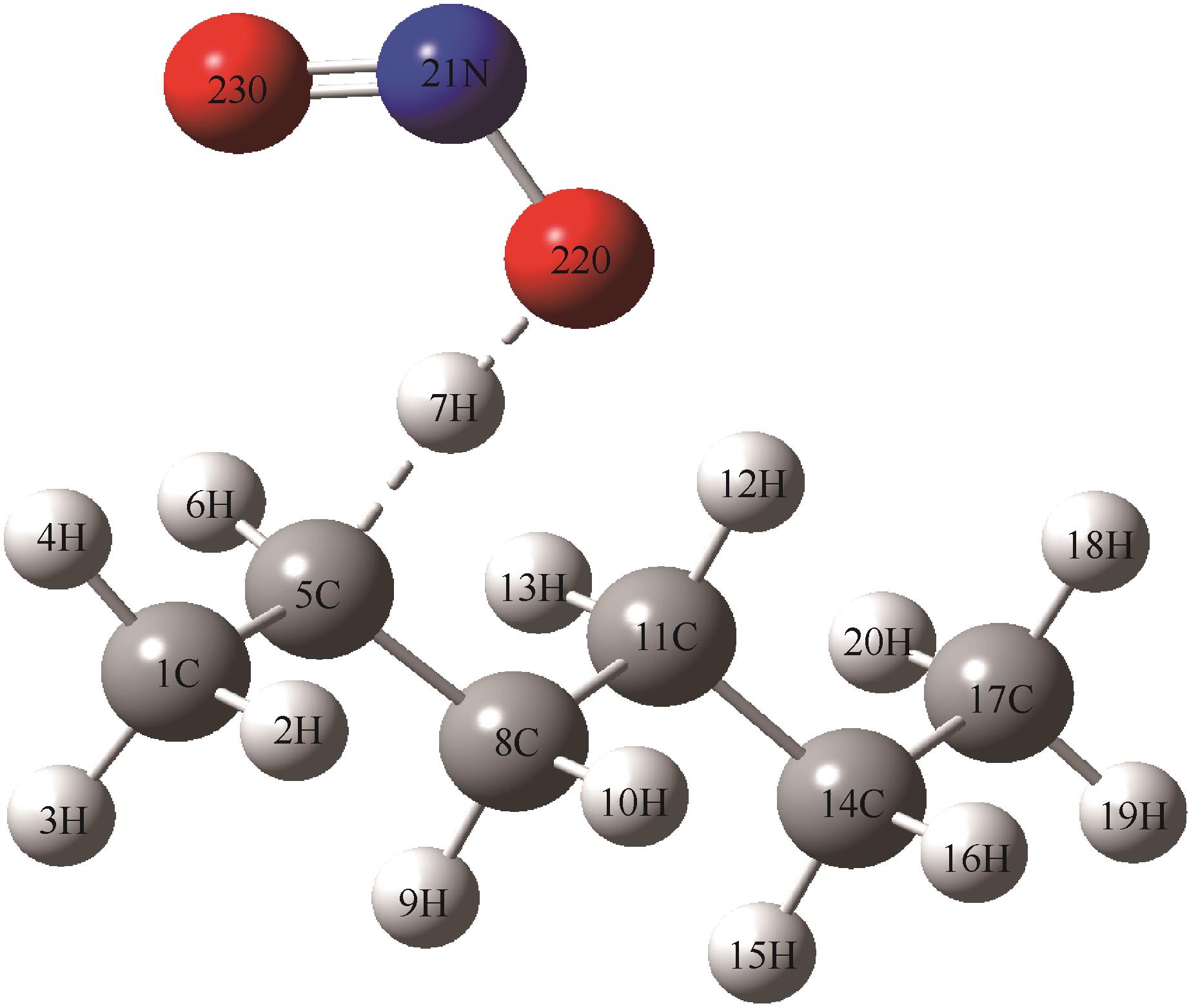
| 几何参数 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) |
|---|---|---|---|
键长/? C(1)—C(5) C(5)—C(8) C(8)—C(11) C(5)—H(6) C(5)—H(7) H(7)—O(22) O(22)—N(21) N(21)—O(23) | 1.531 1.532 1.537 1.097 1.089(0.3525) 1.991(0.0050) 1.205 1.204 | 1.508 1.511 1.536 1.095 1.412(0.1889) 1.190(0.0185) 1.319 1.205 | 1.496 1.499 1.538 1.091 1.988(0.0728) 1.009(0.1121) 1.372 1.197 |
键角/(°) C(1)—C(5)—C(8) C(5)—C(8)—C(11) C(8)—C(11)—C(14) C(11)—C(14)—C(17) C(1)—C(5)—H(7) C(8)—C(5)—H(7) | 115.4 113.0 113.3 113.7 109.0 107.8 | 118.3 114.6 113.2 113.0 104.1 103.2 | 121.6 114.2 113.3 113.1 98.3 100.5 |
二面角/(°) C(5)—N(21)—O(22)—O(23) H(7)—N(21)—O(22)—O(23) | -1.0 -7.7 | -0.9 -1.2 | -0.3 -0.9 |
| 几何参数 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) |
|---|---|---|---|
键长/? C(1)—C(5) C(5)—C(8) C(8)—C(11) C(5)—H(6) C(5)—H(7) H(7)—O(22) O(22)—N(21) N(21)—O(23) | 1.531 1.532 1.537 1.097 1.089(0.3525) 1.991(0.0050) 1.205 1.204 | 1.508 1.511 1.536 1.095 1.412(0.1889) 1.190(0.0185) 1.319 1.205 | 1.496 1.499 1.538 1.091 1.988(0.0728) 1.009(0.1121) 1.372 1.197 |
键角/(°) C(1)—C(5)—C(8) C(5)—C(8)—C(11) C(8)—C(11)—C(14) C(11)—C(14)—C(17) C(1)—C(5)—H(7) C(8)—C(5)—H(7) | 115.4 113.0 113.3 113.7 109.0 107.8 | 118.3 114.6 113.2 113.0 104.1 103.2 | 121.6 114.2 113.3 113.1 98.3 100.5 |
二面角/(°) C(5)—N(21)—O(22)—O(23) H(7)—N(21)—O(22)—O(23) | -1.0 -7.7 | -0.9 -1.2 | -0.3 -0.9 |
| 原子 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) | 原子 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) |
|---|---|---|---|---|---|---|---|
| C(1) | -0.4413 | -0.4516 | -0.4750 | H(13) | 0.1241 | 0.1320 | 0.1320 |
| H(2) | 0.1398 | 0.1604 | 0.1569 | C(14) | -0.2464 | -0.2500 | -0.2497 |
| H(3) | 0.1405 | 0.1624 | 0.1612 | H(15) | 0.1289 | 0.1307 | 0.1311 |
| H(4) | 0.1414 | 0.1758 | 0.1728 | H(16) | 0.1299 | 0.1347 | 0.1343 |
| C(5) | -0.2490 | -0.2535 | -0.1665 | C(17) | -0.4413 | -0.4430 | -0.4431 |
| H(6) | 0.1291 | 0.1722 | 0.1512 | H(18) | 0.1410 | 0.1468 | 0.1468 |
| H(7) | 0.1230 | 0.3422 | 0.4022 | H(19) | 0.1405 | 0.1445 | 0.1442 |
| C(8) | -0.2436 | -0.2546 | -0.2697 | H(20) | 0.1401 | 0.1429 | 0.1424 |
| H(9) | 0.1250 | 0.1509 | 0.1475 | N(21) | 0.4838 | 0.3091 | 0.2935 |
| H(10) | 0.1261 | 0.1484 | 0.1447 | O(22) | -0.2365 | -0.3971 | -0.4660 |
| C(11) | -0.2494 | -0.2572 | -0.2590 | O(23) | -0.2340 | -0.2966 | -0.2765 |
| H(12) | 0.1282 | 0.1508 | 0.1446 |
| 原子 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) | 原子 | 反应物(R) | 过渡态(TS2) | 中间体(IM2) |
|---|---|---|---|---|---|---|---|
| C(1) | -0.4413 | -0.4516 | -0.4750 | H(13) | 0.1241 | 0.1320 | 0.1320 |
| H(2) | 0.1398 | 0.1604 | 0.1569 | C(14) | -0.2464 | -0.2500 | -0.2497 |
| H(3) | 0.1405 | 0.1624 | 0.1612 | H(15) | 0.1289 | 0.1307 | 0.1311 |
| H(4) | 0.1414 | 0.1758 | 0.1728 | H(16) | 0.1299 | 0.1347 | 0.1343 |
| C(5) | -0.2490 | -0.2535 | -0.1665 | C(17) | -0.4413 | -0.4430 | -0.4431 |
| H(6) | 0.1291 | 0.1722 | 0.1512 | H(18) | 0.1410 | 0.1468 | 0.1468 |
| H(7) | 0.1230 | 0.3422 | 0.4022 | H(19) | 0.1405 | 0.1445 | 0.1442 |
| C(8) | -0.2436 | -0.2546 | -0.2697 | H(20) | 0.1401 | 0.1429 | 0.1424 |
| H(9) | 0.1250 | 0.1509 | 0.1475 | N(21) | 0.4838 | 0.3091 | 0.2935 |
| H(10) | 0.1261 | 0.1484 | 0.1447 | O(22) | -0.2365 | -0.3971 | -0.4660 |
| C(11) | -0.2494 | -0.2572 | -0.2590 | O(23) | -0.2340 | -0.2966 | -0.2765 |
| H(12) | 0.1282 | 0.1508 | 0.1446 |
| 频率 | 反应物 | 过渡态 | 中间体 | 频率 | 反应物 | 过渡态 | 中间体 |
|---|---|---|---|---|---|---|---|
| υ1 | 5(0) | -1417(355) | 16(0) | υ33 | 1290(0) | 1209(18) | 1233(8) |
| υ2 | 14(0) | 31(0) | 34(1) | υ34 | 1297(1) | 1231(16) | 1270(1) |
| υ3 | 19(0) | 48(0) | 40(0) | υ35 | 1306(0) | 1272(3) | 1295(0) |
| υ4 | 49(0) | 57(0) | 71(0) | υ36 | 1345(0) | 1294(1) | 1303(1) |
| υ5 | 52(0) | 93(0) | 79(0) | υ37 | 1355(5) | 1299(1) | 1345(15) |
| υ6 | 71(0) | 117(1) | 93(0) | υ38 | 1368(0) | 1341(3) | 1358(3) |
| υ7 | 86(1) | 130(0) | 125(1) | υ39 | 1384(3) | 1358(1) | 1374(1) |
| υ8 | 98(0) | 139(0) | 126(0) | υ40 | 1385(0) | 1369(1) | 1380(8) |
| υ9 | 124(0) | 166(0) | 148(1) | υ41 | 1452(1) | 1374(10) | 1386(3) |
| υ10 | 148(0) | 208(0) | 166(2) | υ42 | 1454(0) | 1385(2) | 1433(1) |
| υ11 | 232(0) | 239(0) | 208(4) | υ43 | 1460(0) | 1433(0) | 1450(5) |
| υ12 | 243(0) | 250(0) | 245(0) | υ44 | 1468(9) | 1450(7) | 1454(10) |
| υ13 | 286(0) | 301(0) | 295(0) | υ45 | 1468(1) | 1457(0) | 1457(0) |
| υ14 | 352(0) | 362(0) | 354(0) | υ46 | 1469(3) | 1459(5) | 1467(0) |
| υ15 | 448(0) | 438(0) | 449(0) | υ47 | 1477(0) | 1467(1) | 1469(6) |
| υ16 | 710(4) | 475(1) | 565(24) | υ48 | 1482(3) | 1469(6) | 1480(3) |
| υ17 | 721(5) | 706(6) | 651(14) | υ49 | 1654(296) | 1480(3) | 1634(177) |
| υ18 | 723(1) | 729(1) | 710(2) | υ50 | 2890(1) | 1571(270) | 2814(28) |
| υ19 | 783(1) | 770(0) | 760(2) | υ51 | 2893(6) | 2844(16) | 2856(30) |
| υ20 | 861(3) | 813(11) | 829(70) | υ52 | 2903(0) | 2890(17) | 2899(1) |
| υ21 | 879(0) | 866(0) | 864(2) | υ53 | 2907(94) | 2901(3) | 2902(4) |
| υ22 | 880(0) | 881(4) | 879(4) | υ54 | 2911(19) | 2907(32) | 2910(61) |
| υ23 | 979(0) | 887(1) | 889(1) | υ55 | 2919(72) | 2921(13) | 2922(34) |
| υ24 | 981(1) | 981(12) | 913(270) | υ56 | 2919(13) | 2923(48) | 2926(3) |
| υ25 | 1017(0) | 989(2) | 965(1) | υ57 | 2920(2) | 2928(3) | 2945(14) |
| υ26 | 1027(0) | 1025(13) | 986(0) | υ58 | 2935(1) | 2953(35) | 2949(43) |
| υ27 | 1042(4) | 1036(16) | 1026(1) | υ59 | 2949(90) | 2963(12) | 2985(56) |
| υ28 | 1122(0) | 1061(83) | 1036(4) | υ60 | 2981(15) | 2984(28) | 2990(41) |
| υ29 | 1173(0) | 1078(3) | 1084(0) | υ61 | 2982(126) | 2985(50) | 2992(7) |
| υ30 | 1217(0) | 1120(19) | 1095(2) | υ62 | 2985(46) | 2990(41) | 3016(44) |
| υ31 | 1235(5) | 1140(10) | 1135(1) | υ63 | 2986(42) | 3012(19) | 3024(381) |
| υ32 | 1272(0) | 1165(29) | 1199(0) |
| 频率 | 反应物 | 过渡态 | 中间体 | 频率 | 反应物 | 过渡态 | 中间体 |
|---|---|---|---|---|---|---|---|
| υ1 | 5(0) | -1417(355) | 16(0) | υ33 | 1290(0) | 1209(18) | 1233(8) |
| υ2 | 14(0) | 31(0) | 34(1) | υ34 | 1297(1) | 1231(16) | 1270(1) |
| υ3 | 19(0) | 48(0) | 40(0) | υ35 | 1306(0) | 1272(3) | 1295(0) |
| υ4 | 49(0) | 57(0) | 71(0) | υ36 | 1345(0) | 1294(1) | 1303(1) |
| υ5 | 52(0) | 93(0) | 79(0) | υ37 | 1355(5) | 1299(1) | 1345(15) |
| υ6 | 71(0) | 117(1) | 93(0) | υ38 | 1368(0) | 1341(3) | 1358(3) |
| υ7 | 86(1) | 130(0) | 125(1) | υ39 | 1384(3) | 1358(1) | 1374(1) |
| υ8 | 98(0) | 139(0) | 126(0) | υ40 | 1385(0) | 1369(1) | 1380(8) |
| υ9 | 124(0) | 166(0) | 148(1) | υ41 | 1452(1) | 1374(10) | 1386(3) |
| υ10 | 148(0) | 208(0) | 166(2) | υ42 | 1454(0) | 1385(2) | 1433(1) |
| υ11 | 232(0) | 239(0) | 208(4) | υ43 | 1460(0) | 1433(0) | 1450(5) |
| υ12 | 243(0) | 250(0) | 245(0) | υ44 | 1468(9) | 1450(7) | 1454(10) |
| υ13 | 286(0) | 301(0) | 295(0) | υ45 | 1468(1) | 1457(0) | 1457(0) |
| υ14 | 352(0) | 362(0) | 354(0) | υ46 | 1469(3) | 1459(5) | 1467(0) |
| υ15 | 448(0) | 438(0) | 449(0) | υ47 | 1477(0) | 1467(1) | 1469(6) |
| υ16 | 710(4) | 475(1) | 565(24) | υ48 | 1482(3) | 1469(6) | 1480(3) |
| υ17 | 721(5) | 706(6) | 651(14) | υ49 | 1654(296) | 1480(3) | 1634(177) |
| υ18 | 723(1) | 729(1) | 710(2) | υ50 | 2890(1) | 1571(270) | 2814(28) |
| υ19 | 783(1) | 770(0) | 760(2) | υ51 | 2893(6) | 2844(16) | 2856(30) |
| υ20 | 861(3) | 813(11) | 829(70) | υ52 | 2903(0) | 2890(17) | 2899(1) |
| υ21 | 879(0) | 866(0) | 864(2) | υ53 | 2907(94) | 2901(3) | 2902(4) |
| υ22 | 880(0) | 881(4) | 879(4) | υ54 | 2911(19) | 2907(32) | 2910(61) |
| υ23 | 979(0) | 887(1) | 889(1) | υ55 | 2919(72) | 2921(13) | 2922(34) |
| υ24 | 981(1) | 981(12) | 913(270) | υ56 | 2919(13) | 2923(48) | 2926(3) |
| υ25 | 1017(0) | 989(2) | 965(1) | υ57 | 2920(2) | 2928(3) | 2945(14) |
| υ26 | 1027(0) | 1025(13) | 986(0) | υ58 | 2935(1) | 2953(35) | 2949(43) |
| υ27 | 1042(4) | 1036(16) | 1026(1) | υ59 | 2949(90) | 2963(12) | 2985(56) |
| υ28 | 1122(0) | 1061(83) | 1036(4) | υ60 | 2981(15) | 2984(28) | 2990(41) |
| υ29 | 1173(0) | 1078(3) | 1084(0) | υ61 | 2982(126) | 2985(50) | 2992(7) |
| υ30 | 1217(0) | 1120(19) | 1095(2) | υ62 | 2985(46) | 2990(41) | 3016(44) |
| υ31 | 1235(5) | 1140(10) | 1135(1) | υ63 | 2986(42) | 3012(19) | 3024(381) |
| υ32 | 1272(0) | 1165(29) | 1199(0) |
| 1 | SIMPSON M F, LAW J D. Nuclear fuel reprocessing[M]//Nuclear Energy. New York: Springer, 2018: 187-204. |
| 2 | 姜圣阶, 任凤仪, 等. 核燃料后处理工学[M]. 北京: 原子能出版社, 1995. |
| JIANG Shengjie, REN Fengyi, et al. Nuclear fuel reprocessing engineering[M]. Beijing: Atomic Energy Press, 1995. | |
| 3 | COLVEN T J, NICHOLS G M, SIDDALL T H. TNX evaporator incident January 12, 1953. interim technical report[R]. Office of Scientific and Technical Information (OSTI), 1953. |
| 4 | DURANT W S. Red oil explosion at the Savannah river plant[R]. DPMS-83-142, Savannah River Laboratory: 1984. |
| 5 | SEGE G. Overconcentration in initial operation of uranium evaporator, 321 Building[R]. Office of Scientific and Technical Information (OSTI), 1953. |
| 6 | DAVIS W J, BALDWIN W H, MESERVEY A B. Chemistry of the intercycle evaporator incident of November 20, 1959[R]. Office of Scientific and Technical Information (OSTI), 1960. |
| 7 | ANDREEV G, ANTIPIN Y, BENNETT B, et al. The radiological accident in the reprocessing plant at Tomsk[R]. International Atomic Energy Agency, Austria: 1998. |
| 8 | MINCHER B J, MODOLO G, MEZYK S P. Review article: the effects of radiation chemistry on solvent extraction: 1. Conditions in acidic solution and a review of TBP radiolysis[J]. Solvent Extraction and Ion Exchange, 2009, 27(1): 1-25. |
| 9 | 许明霞. 乏燃料后处理厂“红油”爆炸安全分析[J]. 核安全, 2011(1): 22-27. |
| XU Mingxia. Safety analysis on explosion of “red oils” in spent fuel reprocessing plants[J]. Nuclear safety, 2011(1): 22-27. | |
| 10 | KUMAR S, KUMAR R, KOGANTI S B. Studies on reaction runaways for Urex/Purex solvent-nitric acid and red-oil synthesis[C]//Oak Ridge National Laboratory,International Solvent Extraction Conference2008. 9ISEC-2008. Tucson, USA. 2008. |
| 11 | KUMAR S, SINHA P K, KAMACHI MUDALI U, et al. Thermal decomposition of red-oil/nitric acid mixtures in adiabatic conditions[J]. Journal of Radioanalytical and Nuclear Chemistry, 2011, 289(2): 545-549. |
| 12 | SMITHA V S, SURIANARAYANAN M, SESHADRI H, et al. Thermal behavior pattern of tributyl phosphate under adiabatic conditions[J]. Journal of Thermal Analysis and Calorimetry, 2013, 111(1): 849-856. |
| 13 | CHANDRAN K, SAHOO T K, MURALIDARAN P, et al. Calorimetric studies on the thermal decomposition of tri n-butyl phosphate-nitric acid systems[J]. Journal of Thermal Analysis and Calorimetry, 2012, 110(2): 879-890. |
| 14 | TALLENT O K, MAILEN J C, DODSON K E. Purex diluent chemical degradation[J]. Nuclear Technology, 1985, 71(2): 417-425. |
| 15 | ISHIHARA T, OHWADA K. Chemical degradation of kerosene diluent with nitric acid[J]. Journal of Nuclear Science and Technology, 2008, 3(1): 20-26. |
| 16 | OHWADA K. On the identification of hydroxamic acids formed by nitric acid degradation of kerosene and i-dodecane[J]. Journal of Nuclear Science and Technology, 1968, 5(4): 163-167. |
| 17 | 周春俐, 王墨, 唐洪彬, 等. 正己烷与正十二烷在硝酸体系中的化学行为[J]. 核化学与放射化学, 2021, 43(2): 122-128. |
| ZHOU Chunli, WANG Mo, TANG Hongbin, et al. Chemical behavior of n-hexane and n-dodecane in nitric acid system[J]. Journal of Nuclear and Radiochemistry, 2021, 43(2): 122-128. | |
| 18 | WAGNER R M. Investigation of explosive characteristics of purex solvent decomposition products (Red oil)[R]. Office of Scientific and Technical Information (OSTI), 1953. |
| 19 | WILBOURN R G. Safety aspects of solvent nitration in HTGR fuel reprocessing[R]. Office of Scientific and Technical Information (OSTI), 1977. |
| 20 | HOU Y, BAREFIELD E K, TEDDER D W, et al. Thermal decomposition of nitrated tributyl phosphate[J]. Nuclear Technology, 1996, 113(3): 304-315. |
| 21 | SAKAGUCHI S, NISHIWAKI Y, KITAMURA T, et al. Efficient catalytic alkane nitration with NO2 under air assisted by N-hydroxyphthalimide[J]. Angewandte Chemie International Edition, 2001, 40(1): 222-224. |
| 22 | NISHIWAKI Y, SAKAGUCHI S, ISHII Y. An efficient nitration of light alkanes and the alkyl side-chain of aromatic compounds with nitrogen dioxide and nitric acid catalyzed by N-hydroxyphthalimide[J]. The Journal of Organic Chemistry, 2002, 67(16): 5663-5668. |
| 23 | SUZUKI H, MURASHIMA T, KOZAI I, et al. Ozone-mediated nitration of alkylbenzenes and related compounds with nitrogen dioxide[J]. Journal of the Chemical Society, Perkin Transactions 1, 1993(14): 1591. |
| 24 | PENG X, SUZUKI H, LU C. Zeolite-assisted nitration of neat toluene and chlorobenzene with a nitrogen dioxide/molecular oxygen system. Remarkable enhancement of para-selectivity[J]. Tetrahedron Letters, 2001, 42(26): 4357-4359. |
| 25 | DONG J, JIN B, SUN P. Palladium-catalyzed direct ortho-nitration of azoarenes using NO2 as nitro source[J]. Organic Letters, 2014, 16(17): 4540-4542. |
| 26 | 许晓娟, 肖鹤鸣, 贡雪东, 等. NO2气相硝化金刚烷的计算研究[J]. 化学学报, 2006, 64(4): 306-312. |
| XU Xiaojuan, XIAO Heming, GONG Xuedong, et al. Computational studies on the nitration of adamantane with NO2[J]. Acta Chimica Sinica, 2006, 64(4): 306-312. |
| [1] | BAI Zhihua, ZHANG Jun. Oxidative removal of NO in DTPMPA/Fenton system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4967-4973. |
| [2] | ZHU Chuanqiang, RU Jinbo, SUN Tingting, XIE Xingwang, LI Changming, GAO Shiqiu. Characteristics of selective non-catalytic reduction of NO x with solid polymer denitration agent [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4939-4946. |
| [3] | LI Dongze, ZHANG Xiang, TIAN Jian, HU Pan, YAO Jie, ZHU Lin, BU Changsheng, WANG Xinye. Research progress of NO x reduction by carbonaceous substances for denitration in cement kiln [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4882-4893. |
| [4] | DENG Jian, WANG Kai, LUO Guangsheng. Development and consideration of adiabatic continuous microreaction technology for safe production of nitro compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3923-3925. |
| [5] | XU Peiyao, CHEN Biaoqi, KANKALA Ranjith Kumar, WANG Shibin, CHEN Aizheng. Research progress of nanomaterials for synergistic ferroptosis anticancer therapy [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3684-3694. |
| [6] | WANG Jiaxin, PAN Yong, XIONG Xinyi, WAN Xiaoyue, WANG Jianchao. Reaction process and hazards of dinitrotoluene preparation by one-step catalytic nitration of toluene [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3420-3430. |
| [7] | LI Dongxian, WANG Jia, JIANG Jianchun. Producing biofuels from soapstock via pyrolysis and subsequent catalytic vapor-phase hydrotreating process [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2874-2883. |
| [8] | LI Yun, CUI Nan, XIONG Xingxing, HUANG Zhiyuan, WANG Dongliang, XU Dan, LI Jun, LI Zebing. Influence of rare earth element Er(Ⅲ) on performance of short-cut nitrification and its inhibition kinetics [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1659-1668. |
| [9] | WU Yiheng, ZHANG Yaoyuan, WU Qin, SHI Daxin, CHEN Kangcheng, LI Hansheng. Research progress of gallium modified HZSM-5 catalysts for aromatization of light alkanes [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5162-5178. |
| [10] | WANG Qinghong, JIANG Chenxu, WANG Xin, YU Meiqi, ZHU Shuai, LI Yiming, CHEN Chunmao. An overview of natural mineral catalytic oxidation of refractory organic contaminants in wastewater [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 417-434. |
| [11] | CHENG Peng, ZHAO Shanshan, YANG Wenlong, QI Yue, DING Xiaoshu, YANG Qiusheng, ZHANG Dongsheng, WANG Yanji. Research progress of 1,5- diaminonaphthalene synthesis [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5011-5021. |
| [12] | ZHANG Xiaoxiao, SHANG Zhenhua, ZHANG Xiangjing. Continuous flow synthesis of nitrochlorobenzene in microtubular reactor [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5022-5028. |
| [13] | MA Jing, MA Ziran, LIN Dehai, MA Shaodan, WANG Baodong. Effect of cosolvent in activation solution on regenerated denitration catalysts [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4173-4180. |
| [14] | PAN Jie, WANG Mingxin, GAO Shengwang, XIA Xunfeng, HAN Xue. Nitrogen-sulfur doped biochar/permonosulfate for degradation of sulfisoxazole in water [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4204-4212. |
| [15] | YI Xuenong, LI Jingmei, GAO Yuqiong. Oxidative degradation of naproxen in water by UV-Fe(Ⅵ) process [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4562-4570. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||