Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (5): 2581-2592.DOI: 10.16085/j.issn.1000-6613.2020-1243
• Industrial catalysis • Previous Articles Next Articles
Zn(Al)O composite oxides supported Au catalysts for selective oxidation of glycerol to 1, 3-dihydroxyacetone
KE Yihu1( ), LI Jingyun1, LIU Chunling2, DONG Wensheng2, LIU Hai1
), LI Jingyun1, LIU Chunling2, DONG Wensheng2, LIU Hai1
- 1.Key Laboratory of Chemical Engineering and Technology, State Ethnic Affairs Commission, School of Chemistry and Chemical Engineering, North Minzu University, Yinchuan 750021, Ningxia, China
2.Key Laboratory of Applied Surface and Colloid Chemistry (SNNU), MOE, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi’an 710119, Shaanxi, China
-
Received:2020-07-02Online:2021-05-24Published:2021-05-06 -
Contact:KE Yihu
Zn(Al)O复合氧化物负载Au催化剂催化氧化甘油制备1,3-二羟基丙酮
- 1.北方民族大学化学与化学工程学院,化工技术基础国家民委重点实验室,宁夏 银川 750021
2.陕西师范大学化学化工学院,应用表面与胶体化学教育部重点实验室,陕西 西安 710119
-
通讯作者:柯义虎 -
作者简介:柯义虎(1984—),男,博士,讲师,硕士生导师,研究方向为多相催化。E-mail:keyihu123@nmu.edu.cn 。 -
基金资助:国家自然科学基金(21862001);宁夏自然科学基金(2019AAC03115);宁夏低品质资源高值化利用技术研发人才小高地;北方民族大学校级重大专项(ZDZX201803)
CLC Number:
Cite this article
KE Yihu, LI Jingyun, LIU Chunling, DONG Wensheng, LIU Hai. Zn(Al)O composite oxides supported Au catalysts for selective oxidation of glycerol to 1, 3-dihydroxyacetone[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2581-2592.
柯义虎, 李景云, 刘春玲, 董文生, 刘海. Zn(Al)O复合氧化物负载Au催化剂催化氧化甘油制备1,3-二羟基丙酮[J]. 化工进展, 2021, 40(5): 2581-2592.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1243
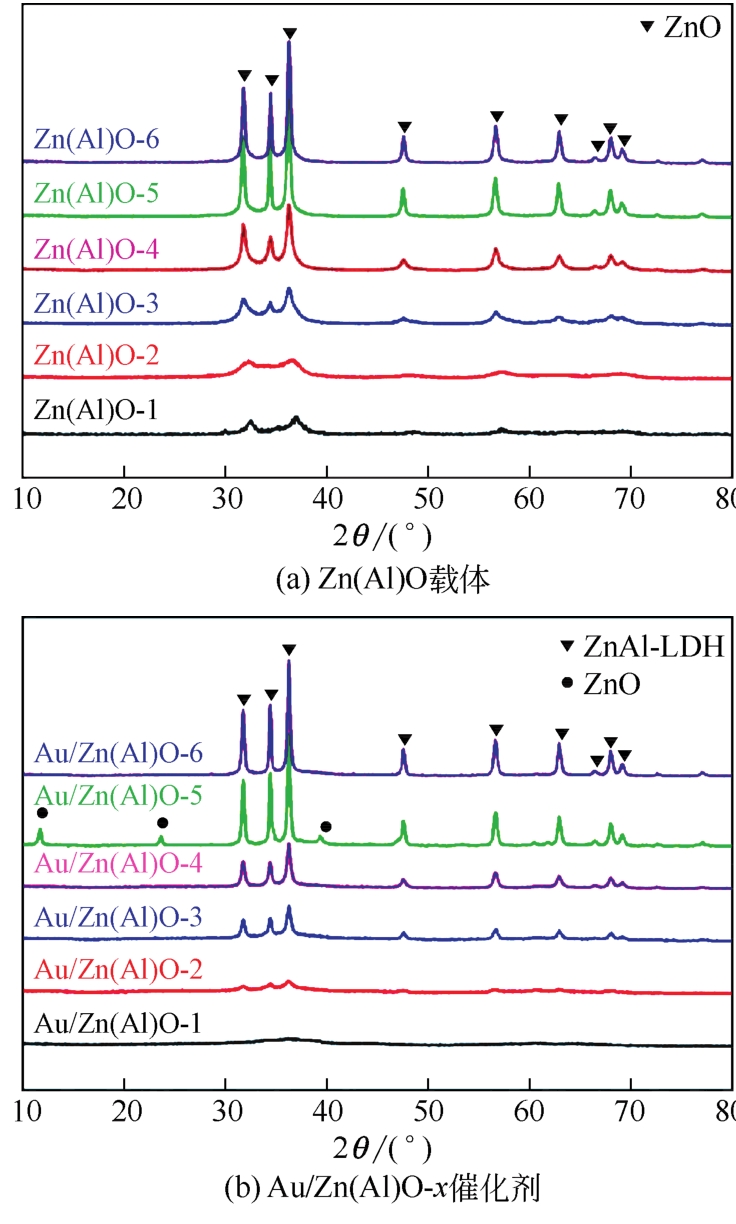
| 催化剂 | Au负载量/% | Zn质量分数/% | Al质量分数/% | Au/Al原子比 | Zn/Al原子比 | SBET /m2·g-1 | Vp/m3·g-1 | Dp/nm |
|---|---|---|---|---|---|---|---|---|
| Au/Zn(Al)O-1 | 1.37 | 33.4 | 12.8 | 0.015 | 1.08 | 113.8 | 0.16 | 7.7 |
| Au/Zn(Al)O-2 | 1.21 | 41.1 | 7.6 | 0.022 | 2.23 | 66.2 | 0.21 | 13.6 |
| Au/Zn(Al)O-3 | 1.15 | 46.5 | 5.6 | 0.028 | 3.45 | 37.7 | 0.20 | 22.3 |
| Au/Zn(Al)O-4 | 1.33 | 54.0 | 3.6 | 0.051 | 6.23 | 34.9 | 0.21 | 25.0 |
| Au/Zn(Al)O-5 | 1.43 | 60.2 | 2.6 | 0.075 | 9.58 | 30.7 | 0.17 | 22.5 |
| Au/Zn(Al)O-6 | 1.14 | 64.9 | 2.2 | 0.071 | 12.25 | 28.9 | 0.17 | 22.9 |
| 催化剂 | Au负载量/% | Zn质量分数/% | Al质量分数/% | Au/Al原子比 | Zn/Al原子比 | SBET /m2·g-1 | Vp/m3·g-1 | Dp/nm |
|---|---|---|---|---|---|---|---|---|
| Au/Zn(Al)O-1 | 1.37 | 33.4 | 12.8 | 0.015 | 1.08 | 113.8 | 0.16 | 7.7 |
| Au/Zn(Al)O-2 | 1.21 | 41.1 | 7.6 | 0.022 | 2.23 | 66.2 | 0.21 | 13.6 |
| Au/Zn(Al)O-3 | 1.15 | 46.5 | 5.6 | 0.028 | 3.45 | 37.7 | 0.20 | 22.3 |
| Au/Zn(Al)O-4 | 1.33 | 54.0 | 3.6 | 0.051 | 6.23 | 34.9 | 0.21 | 25.0 |
| Au/Zn(Al)O-5 | 1.43 | 60.2 | 2.6 | 0.075 | 9.58 | 30.7 | 0.17 | 22.5 |
| Au/Zn(Al)O-6 | 1.14 | 64.9 | 2.2 | 0.071 | 12.25 | 28.9 | 0.17 | 22.9 |
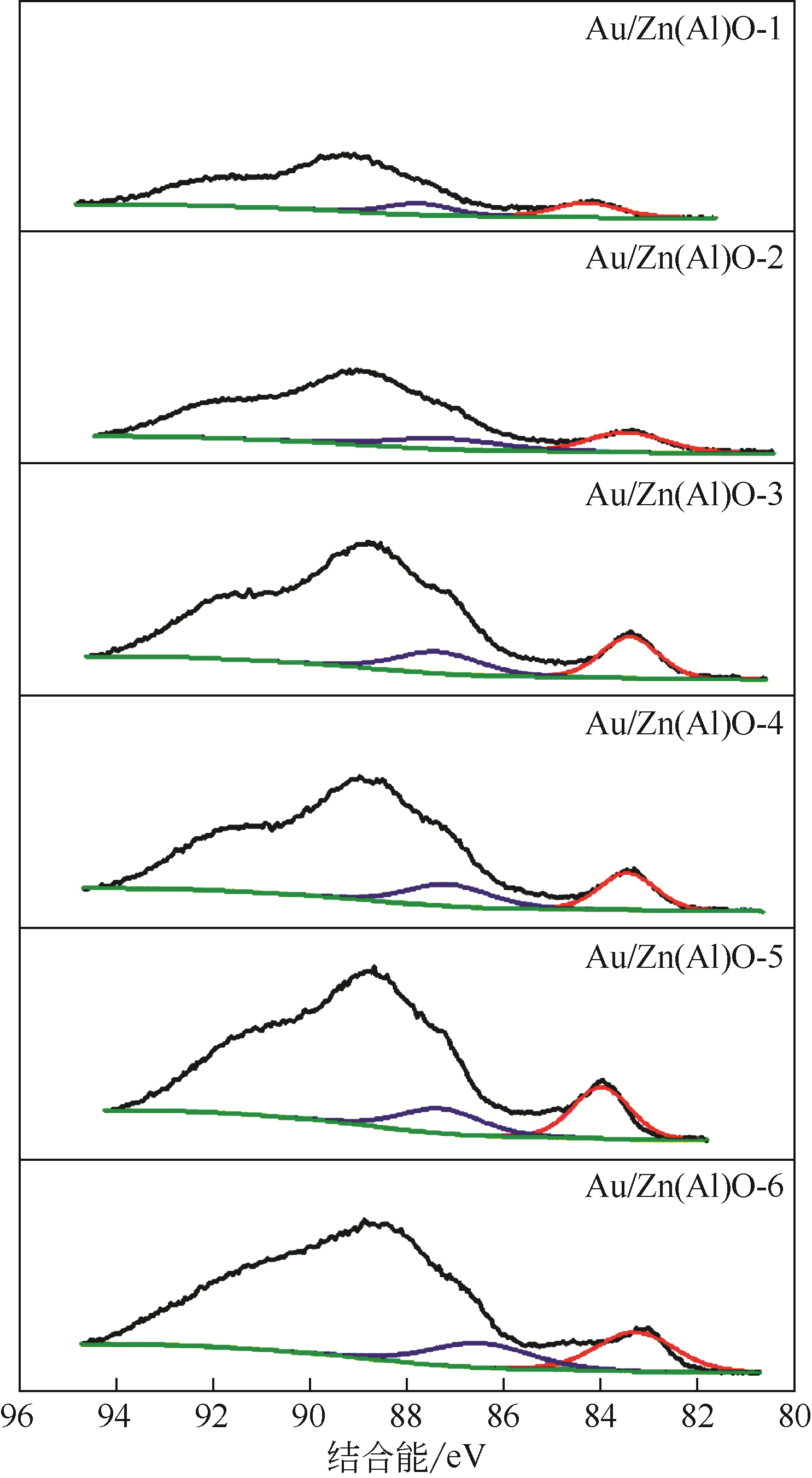
| 催化剂 | Au 4f7/2结合能/eV | Zn 2p3/2结合能/eV | Al 2p3/2结合能/eV | O 1s结合能/eV | Au/Al原子比 | Zn/Al原子比 |
|---|---|---|---|---|---|---|
| Au/Zn(Al)O-1 | 83.57 | 1021.86 | 74.01 | 530.61 | 0.023 | 0.148 |
| Au/Zn(Al)O-2 | 83.48 | 1021.73 | 74.15 | 531.84 | 0.044 | 0.294 |
| Au/Zn(Al)O-3 | 83.41 | 1021.73 | 74.19 | 530.13 | 0.087 | 0.583 |
| Au/Zn(Al)O-4 | 83.43 | 1021.71 | 74.33 | 530.15 | 0.180 | 1.060 |
| Au/Zn(Al)O-5 | 83.51 | 1021.83 | 74.55 | 530.25 | 1.431 | 9.73 |
| Au/Zn(Al)O-6 | 83.54 | 1021.64 | 74.70 | 530.10 | 0.102 | 1.172 |
| 催化剂 | Au 4f7/2结合能/eV | Zn 2p3/2结合能/eV | Al 2p3/2结合能/eV | O 1s结合能/eV | Au/Al原子比 | Zn/Al原子比 |
|---|---|---|---|---|---|---|
| Au/Zn(Al)O-1 | 83.57 | 1021.86 | 74.01 | 530.61 | 0.023 | 0.148 |
| Au/Zn(Al)O-2 | 83.48 | 1021.73 | 74.15 | 531.84 | 0.044 | 0.294 |
| Au/Zn(Al)O-3 | 83.41 | 1021.73 | 74.19 | 530.13 | 0.087 | 0.583 |
| Au/Zn(Al)O-4 | 83.43 | 1021.71 | 74.33 | 530.15 | 0.180 | 1.060 |
| Au/Zn(Al)O-5 | 83.51 | 1021.83 | 74.55 | 530.25 | 1.431 | 9.73 |
| Au/Zn(Al)O-6 | 83.54 | 1021.64 | 74.70 | 530.10 | 0.102 | 1.172 |
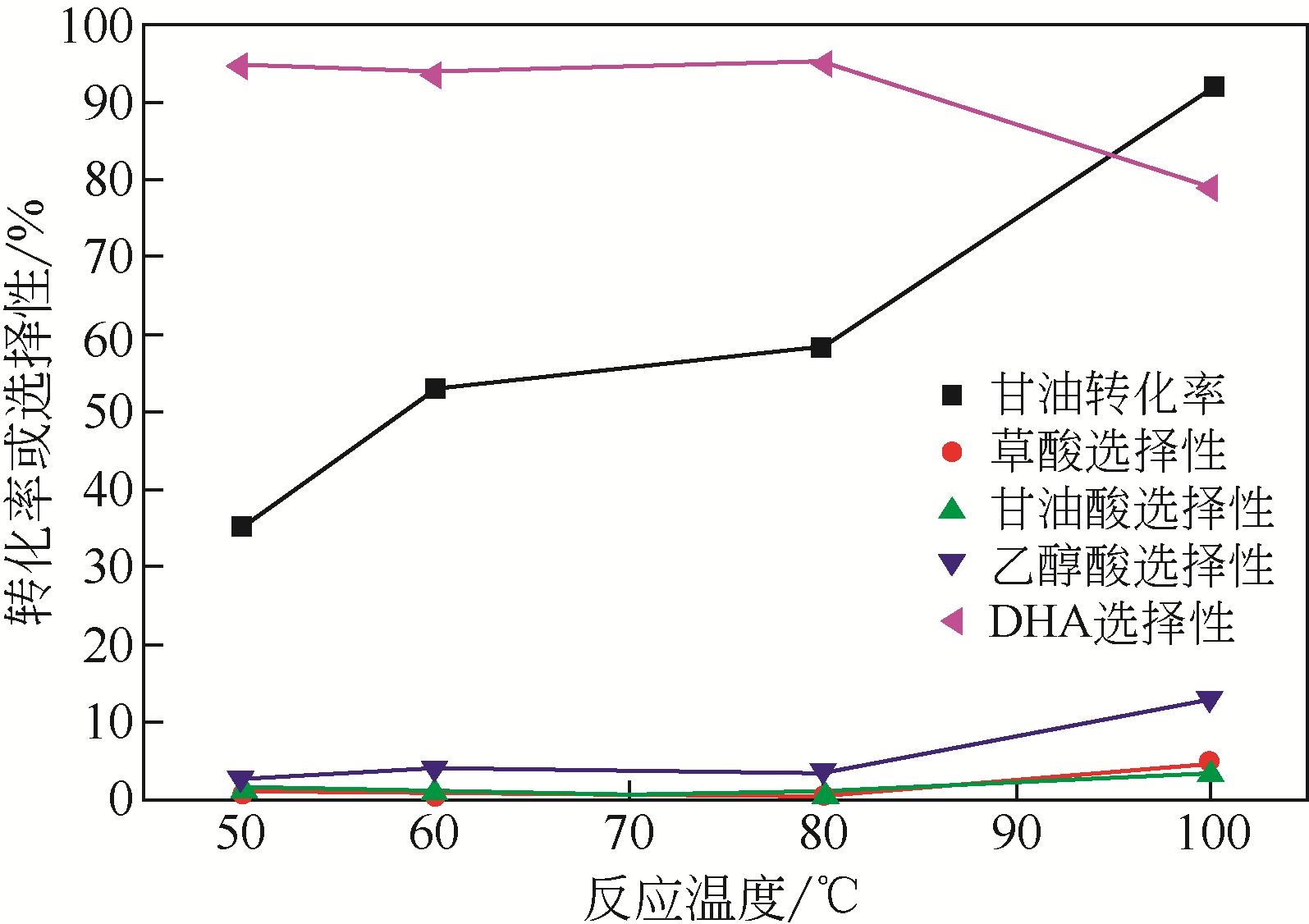
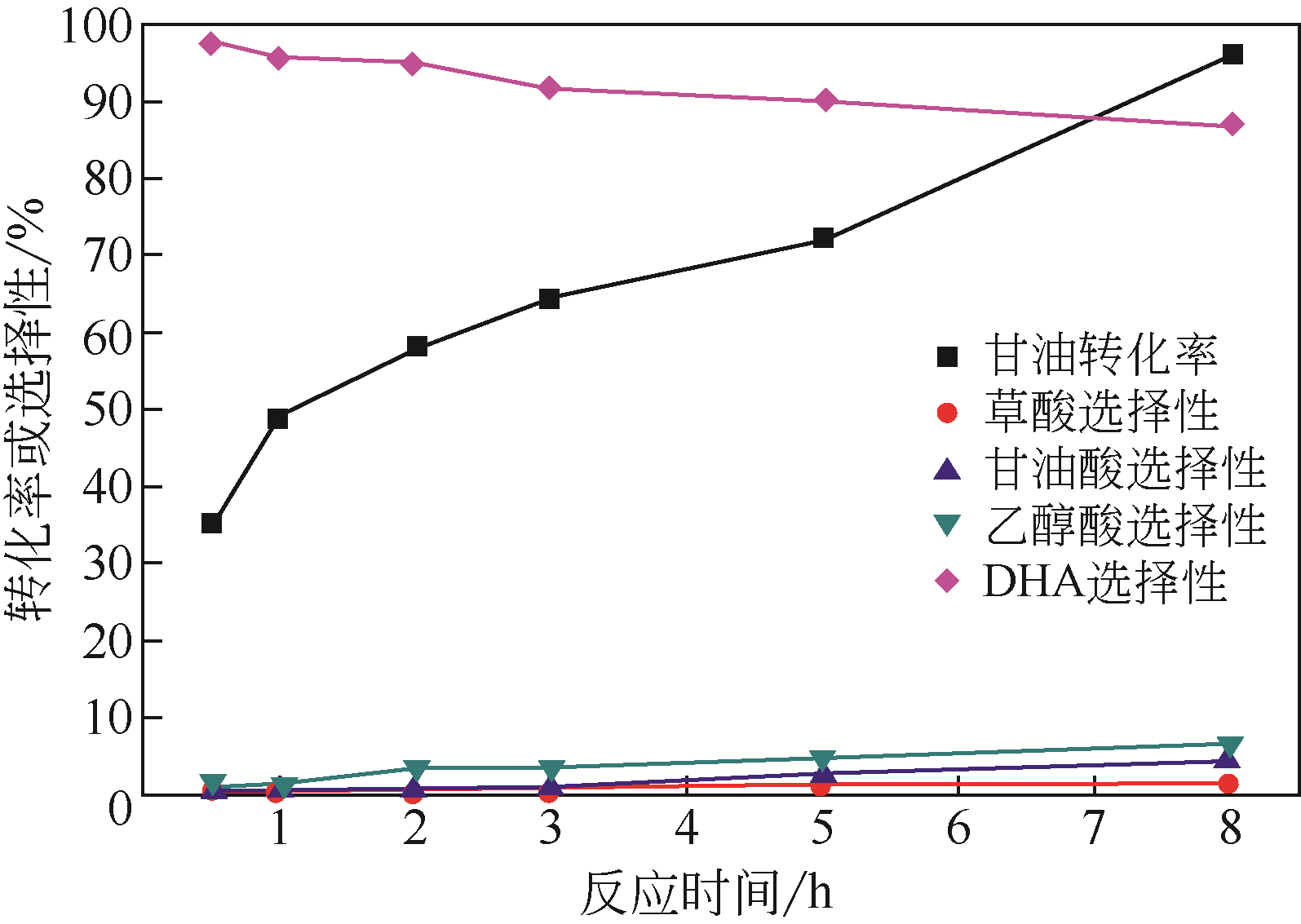
| 催化剂 | 转化率 /% | 选择性/% | 转换频率 /s-1 | |||
|---|---|---|---|---|---|---|
| 草酸 | 甘油酸 | 乙醇酸 | DHA | |||
| Au/Zn(Al)O-1 | 28.9 | 1.8 | 2.4 | 2.4 | 93.4 | 0.0143 |
| Au/Zn(Al)O-2 | 32.6 | 0.8 | 4.5 | 5.6 | 89.1 | 0.0339 |
| Au/Zn(Al)O-3 | 48.2 | 0.6 | 2.0 | 5.8 | 91.6 | 0.0343 |
| Au/Zn(Al)O-4 | 51.3 | 0.6 | 1.6 | 5.7 | 92.2 | 0.0302 |
| Au/Zn(Al)O-5 | 58.5 | 0.4 | 0.9 | 3.4 | 95.3 | 0.0393 |
| Au/Zn(Al)O-6 | 54.4 | 0.5 | 1.2 | 5.0 | 93.3 | 0.0459 |
| 催化剂 | 转化率 /% | 选择性/% | 转换频率 /s-1 | |||
|---|---|---|---|---|---|---|
| 草酸 | 甘油酸 | 乙醇酸 | DHA | |||
| Au/Zn(Al)O-1 | 28.9 | 1.8 | 2.4 | 2.4 | 93.4 | 0.0143 |
| Au/Zn(Al)O-2 | 32.6 | 0.8 | 4.5 | 5.6 | 89.1 | 0.0339 |
| Au/Zn(Al)O-3 | 48.2 | 0.6 | 2.0 | 5.8 | 91.6 | 0.0343 |
| Au/Zn(Al)O-4 | 51.3 | 0.6 | 1.6 | 5.7 | 92.2 | 0.0302 |
| Au/Zn(Al)O-5 | 58.5 | 0.4 | 0.9 | 3.4 | 95.3 | 0.0393 |
| Au/Zn(Al)O-6 | 54.4 | 0.5 | 1.2 | 5.0 | 93.3 | 0.0459 |
| 催化剂 | 转化率/% | 选择性/% | |||
|---|---|---|---|---|---|
| 草酸 | 甘油酸 | 乙醇酸 | DHA | ||
| Au/Zn(Al)O-5-400 | 42.0 | 0.5 | 2.0 | 4.6 | 93.0 |
| Au/Zn(Al)O-5-500 | 58.5 | 0.4 | 0.9 | 3.4 | 95.3 |
| Au/Zn(Al)O-5-600 | 47.9 | 0.4 | 1.7 | 6.4 | 91.4 |
| 催化剂 | 转化率/% | 选择性/% | |||
|---|---|---|---|---|---|
| 草酸 | 甘油酸 | 乙醇酸 | DHA | ||
| Au/Zn(Al)O-5-400 | 42.0 | 0.5 | 2.0 | 4.6 | 93.0 |
| Au/Zn(Al)O-5-500 | 58.5 | 0.4 | 0.9 | 3.4 | 95.3 |
| Au/Zn(Al)O-5-600 | 47.9 | 0.4 | 1.7 | 6.4 | 91.4 |
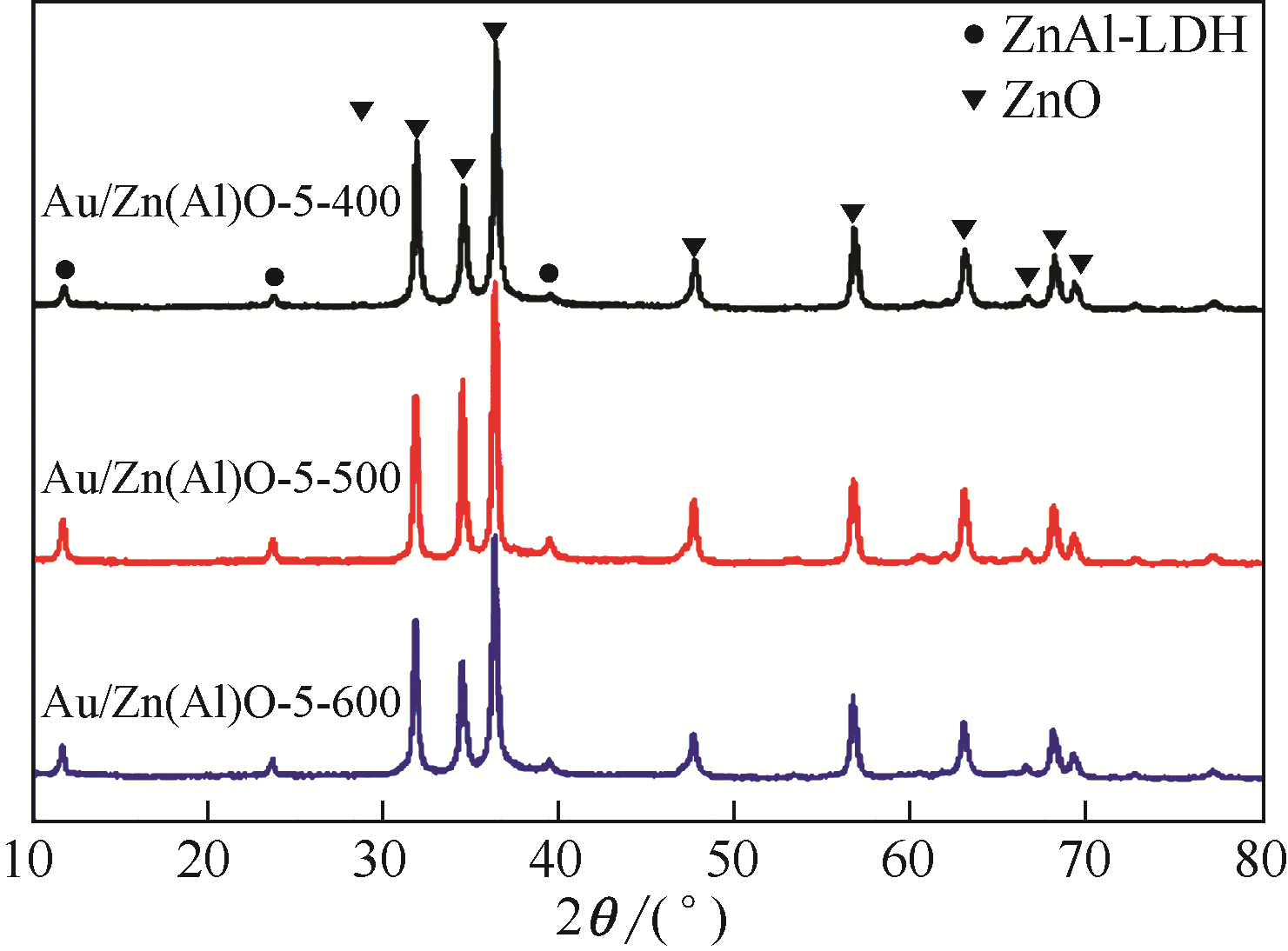
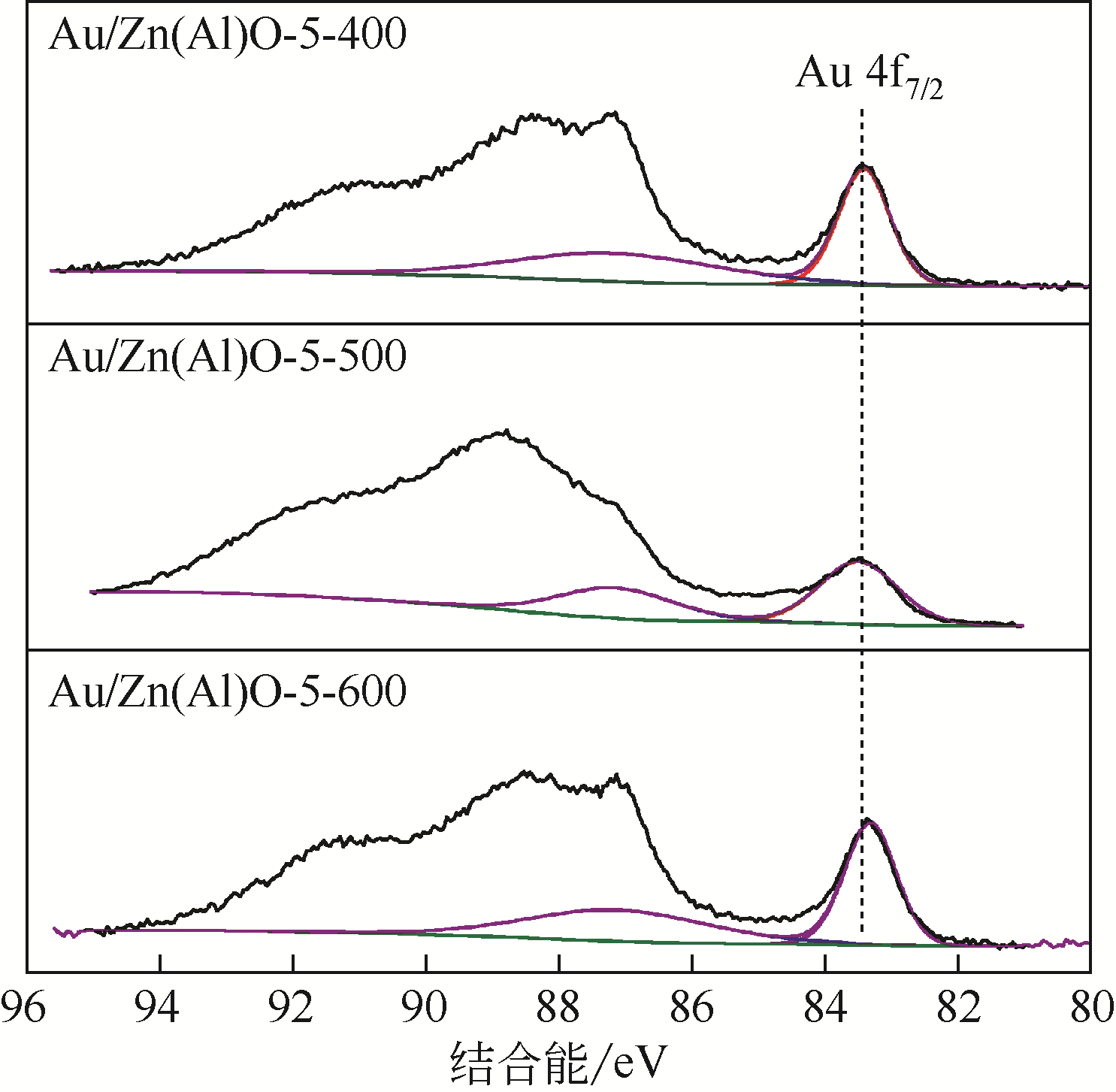
| 催化剂 | Au 4f7/2结合能/eV | Zn 2p3/2结合能/eV | Al 2p3/2结合能/eV | Au/Al原子比 | Zn/Al原子比 | SBET/m2·g-1 | Vp/m3·g-1 | Dp/nm |
|---|---|---|---|---|---|---|---|---|
| Au/Zn(Al)O-5-400 | 83.43 | 1021.52 | 74.54 | 1.439 | 9.68 | 36.7 | 0.18 | 19.5 |
| Au/Zn(Al)O-5-500 | 83.51 | 1021.83 | 74.55 | 1.431 | 9.73 | 30.7 | 0.17 | 22.5 |
| Au/Zn(Al)O-5-600 | 83.35 | 1021.59 | 74.53 | 1.420 | 9.85 | 34.0 | 0.19 | 22.4 |
| 催化剂 | Au 4f7/2结合能/eV | Zn 2p3/2结合能/eV | Al 2p3/2结合能/eV | Au/Al原子比 | Zn/Al原子比 | SBET/m2·g-1 | Vp/m3·g-1 | Dp/nm |
|---|---|---|---|---|---|---|---|---|
| Au/Zn(Al)O-5-400 | 83.43 | 1021.52 | 74.54 | 1.439 | 9.68 | 36.7 | 0.18 | 19.5 |
| Au/Zn(Al)O-5-500 | 83.51 | 1021.83 | 74.55 | 1.431 | 9.73 | 30.7 | 0.17 | 22.5 |
| Au/Zn(Al)O-5-600 | 83.35 | 1021.59 | 74.53 | 1.420 | 9.85 | 34.0 | 0.19 | 22.4 |
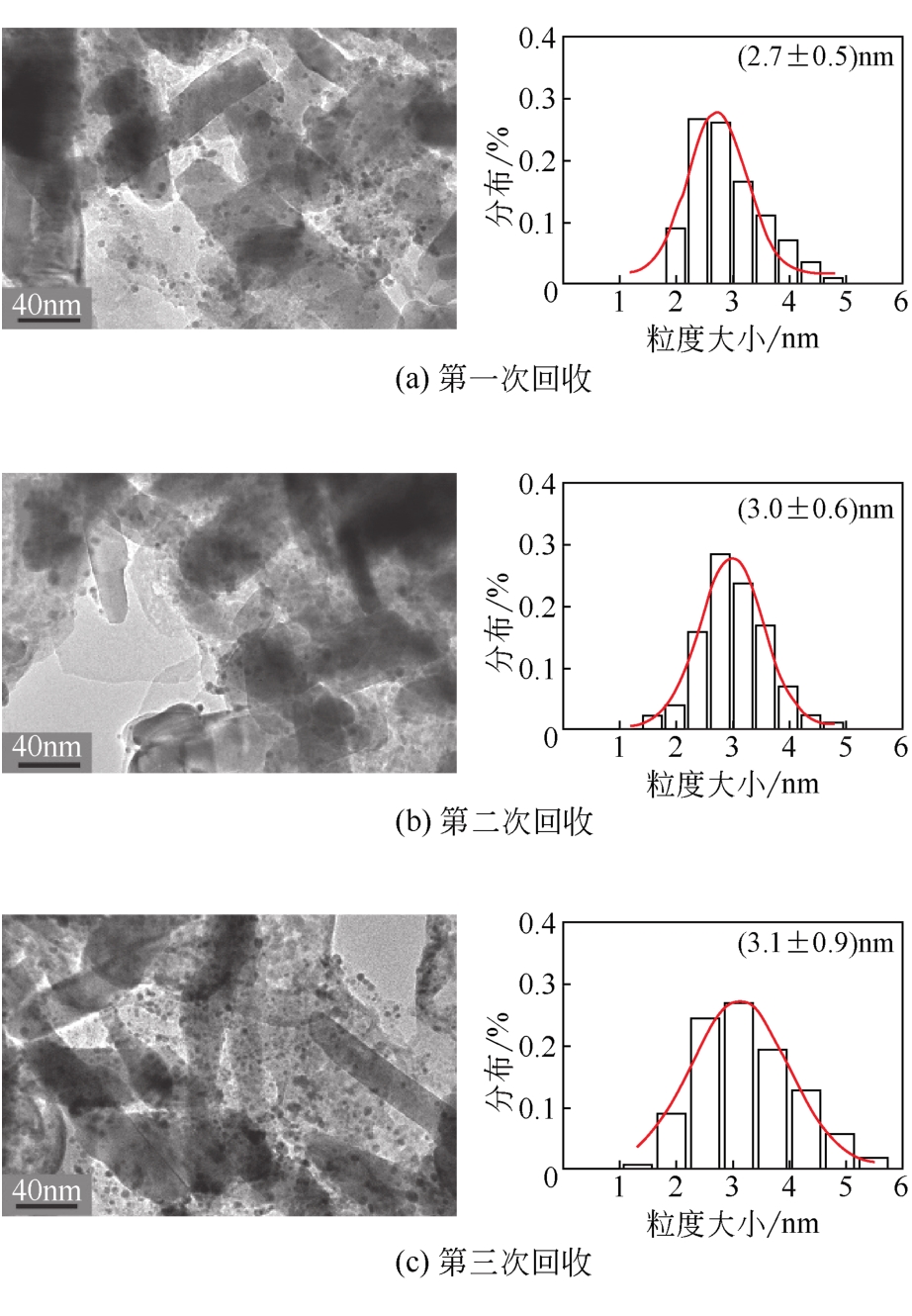
反应 次数 | 转化率 /% | 选择性/% | Zn元素流失 /% | |||
|---|---|---|---|---|---|---|
| 草酸 | 甘油酸 | 乙醇酸 | DHA | |||
| 1 | 58.5 | 0.4 | 0.9 | 3.4 | 95.3 | 3.41 |
| 2 | 17.3 | 0.6 | 5.2 | 3.6 | 90.7 | 2.20 |
| 3 | 9.5 | 0.9 | 7.1 | 2.3 | 89.7 | 1.74 |
反应 次数 | 转化率 /% | 选择性/% | Zn元素流失 /% | |||
|---|---|---|---|---|---|---|
| 草酸 | 甘油酸 | 乙醇酸 | DHA | |||
| 1 | 58.5 | 0.4 | 0.9 | 3.4 | 95.3 | 3.41 |
| 2 | 17.3 | 0.6 | 5.2 | 3.6 | 90.7 | 2.20 |
| 3 | 9.5 | 0.9 | 7.1 | 2.3 | 89.7 | 1.74 |
| 1 | Amin TALEBIAN-KIAKALAIEH, AMIN Nor Aishah Saidina, RAJAEI Kourosh, et al. Oxidation of bio-renewable glycerol to value-added chemicals through catalytic and electro-chemical processes[J]. Applied Energy, 2018, 230: 1347-1379. |
| 2 | VILLA Alberto, DIMITRATOS Nikolaos, CHAN-THAW Carine E, et al. Glycerol oxidation using gold-containing catalysts[J]. Accounts of Chemical Research, 2015, 48(5): 1403-1412. |
| 3 | CORMA Avelino, IBORRA Sara, VELTY Alexandra. Chemical routes for the transformation of biomass into chemicals[J]. Chemical Reviews, 2007, 107(6): 2411-2502. |
| 4 | BEHR Arno, EILTING Jens, IRAWADI Ken, et al. Improved utilisation of renewable resources: new important derivatives of glycerol[J]. Green Chemistry, 2008, 10(1): 13-30. |
| 5 | ZHOU Chunhui, BELTRAMINI Jorge N, FAN Yongxian, et al. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals[J]. Chemical Society Reviews, 2008, 37(3): 527-549. |
| 6 | CATTERTTIN Silvio, MCMORN Paul, JOHNSTON Peter, et al. Selective oxidation of glycerol to glyceric acid using a gold catalyst in aqueous sodium hydroxide[J]. Chemical Communications, 2002(7): 696-697. |
| 7 | DEMIREL Silvio, LEHNERT K, LUCAS M, et al. Use of renewables for the production of chemicals: glycerol oxidation over carbon supported gold catalysts[J]. Applied Catalysis B: Environmental, 2007, 70(1-4): 637-643. |
| 8 | BESSON M, GALLEZOT P, PINEL G. Conversion of biomass into chemicals over metal catalysts[J]. Chemical Reviews, 2014, 114(3): 1827-1870. |
| 9 | DODEKATOS Godekatos, Stefan SCHÜINENMANN, Harun TÜYSÜZ. Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation[J]. ACS Catalysis, 2018, 8(7): 6301-6333. |
| 10 | YANG Lihua, LI Xuewen, CHEN Ping, et al. Selective oxidation of glycerol in a base-free aqueous solution: a short review[J]. Chinese Journal of Catalysis, 2019, 40(7): 1020-1034. |
| 11 | KATRYNIOK Benjamin, KIMURA Hiroshi, Elżbieta SKRZYŃSKA, et al. Selective catalytic oxidation of glycerol: perspectives for high value chemicals[J]. Green Chemistry, 2011, 13(8): 1960-1979. |
| 12 | Juraj ŠVITEL, Ernest ŠTURDÍK. Product yield and by-product formation in glycerol conversion to dihydroxyacetone by Gluconobacter oxydans[J]. Journal of Fermentation and Bioengineering, 1994, 78(5): 351-355. |
| 13 | WEI Shenghua, SONG Qingxun, WEI Dongzhi. Production of Gluconobacter oxydans cells from low-cost culture medium for conversion of glycerol to dihydroxyacetone[J]. Preparative Biochemistry & Biotechnology, 2007, 37(2):113-121. |
| 14 | KIMURA H, TSUTO K, WAKISAKA T, et al. Selective oxidation of glycerol on a platinum-bismuth catalyst[J]. Applied Catalysis A: General, 1993, 96 (2): 217-228. |
| 15 | LIANG Dan, GAO Jing, WANG Junhua, et al. Selective oxidation of glycerol in a base-free aqueous solution over different sized Pt catalysts[J]. Catalysis Communications, 2009, 10(12): 1586-1590. |
| 16 | GAO Jing, LIANG Dan, CHEN Ping, et al. Oxidation of glycerol with oxygen in a base-free aqueous solution over Pt/AC and Pt/MWNTs catalysts[J]. Catalysis Letters, 2009, 130(1/2): 185-191. |
| 17 | LIANG Dan, GAO Jing, SUN Hui, et al. Selective oxidation of glycerol with oxygen in a base-free aqueous solution over MWNTs supported Pt catalysts[J]. Applied Catalysis B: Environmental, 2011, 106: 423-432. |
| 18 | CHEN Shasha, QI Puyu, CHEN Jin, et al. Platinum nanoparticles supported on N-doped carbon nanotubes for the selective oxidation of glycerol to glyceric acid in a base-free aqueous solution[J]. RSC Advances, 2015, 5: 31566-31574. |
| 19 | ZHANG Mengyuan, SHI Juanjuan, SUN Yanyan, et al. Selective oxidation of glycerol over nitrogen-doped carbon nanotubes supported platinum catalyst in base-free solution[J]. Catalysis Communications, 2015, 70: 72-76. |
| 20 | KIMURA Hiroshi. Selective oxidation of glycerol on a platinum-bismuth catalyst by using a fixed bed reactor[J]. Applied Catalysis A: General, 1993, 105(2): 147-158. |
| 21 | GARCIA Régis, BESSON Michèle, GALLEZOT Pierre. Chemoselective catalytic oxidation of glycerol with air on platinum metals[J]. Applied Catalysis A: General, 1995, 127(1/2): 165-176. |
| 22 | HU Wenbin, KNIGHT Daniel, LOWRY Brian, et al. Selective oxidation of glycerol to dihydroxyacetone over Pt-Bi/C catalyst: optimization of catalyst and reaction conditions[J]. Industrial & Engineering Chemistry Research, 2010, 49(21): 10876-10882. |
| 23 | BRANDNER A, LEHNERT K, BIENHOLZ A, et al. Production of biomass-derived chemicals and energy: chemocatalytic conversions of glycerol[J]. Topics in Catalysis, 2009, 52: 278-287. |
| 24 | LIANG Dan, CUI Shiyu, GAO Jing, et al. Glycerol oxidation with oxygen over bimetallic Pt-Bi catalysts under atmospheric pressure[J]. Chinese Journal of Catalysis, 2011, 32(11/12): 1831-1837. |
| 25 | NIE Renfeng, LIANG Dan, SHEN Lian, et al. Selective oxidation of glycerol with oxygen in base-free solution over MWCNTs supported PtSb alloy nanoparticles[J]. Applied Catalysis B: Environmental, 2012, 127: 212-220. |
| 26 | DOU Jian, ZHANG Bowei, LIU Hai, et al. Carbon supported Pt9Sn1 nanoparticles as an efficient nanocatalyst for glycerol oxidation[J]. Applied Catalysis B: Environmental, 2016, 180: 78-85. |
| 27 | HIRASAWA S, NAKAGAWA Y, TOMISHIGE K. Selective oxidation of glycerol to dihydroxyacetone over a Pd-Ag catalyst[J]. Catalysis Science & Technology, 2012, 2: 1150-1152. |
| 28 | HIRASAWA S, WATANABE H, KIZUKA T, et al. Performance, structure and mechanism of Pd-Ag alloy catalyst for selective oxidation of glycerol to dihydroxyacetone[J]. Journal of Catalysis, 2013, 300: 205-216. |
| 29 | HARUTA Masatake, KOBAYASHI Tetsuhiko, SANO Hiroshi, et al. Novel gold catalysts for the oxidation of carbon-monoxide at a temperature far below 0℃[J]. Chemistry Letters, 1987, 16(2): 405-408. |
| 30 | Núria LOPEZ, Mónica GARCIA-MOTA, Jaime GOMEZ-DIAZ. NH3 oxidation on oxygen-precovered Au(111): a density functional theory study on selectivity[J]. The Journal of Physical Chemistry C, 2008, 112: 247-252. |
| 31 | XU Bingjun, LIU Xiaoying, HAUBRICH Jan, et al. Selectivity control in gold-mediated esterification of methanol[J]. Angewandte Chemie: International Edition, 2009, 48: 4206-4209. |
| 32 | OUTKA Duane A, MADIX Robert J. Broensted basicity of atomic oxygen on the gold(110) surface: reactions with methanol, acetylene, water, and ethylene[J]. Journal of the American Chemical Society, 1987, 109: 1708-1714. |
| 33 | HE Lin, NI Ji, WANG Lucun, et al. Aqueous room-temperature gold-catalyzed chemoselective transfer hydrogenation of aldehydes[J]. Chemistry, 2009, 15(44):11833-11836. |
| 34 | DU Xianlong, HE Lin, ZHAO She, et al. Hydrogen-independent reductive transformation of carbohydrate biomass into γ-valeroiactone and pyrrolidone derivatives with supported gold catalysts[J]. Angewandte Chemie: International Edition, 2011, 50(34): 7815-7819. |
| 35 | HE Lin, WANG Lucun, SUN Hao, et al. Efficient and selective room-temperature gold-catalyzed reduction of nitro compounds with CO and H2O as the hydrogen source[J]. Angewandte Chemie International Edition, 2009, 48(50): 9538-9541. |
| 36 | RODRIGUES Elodie G, PEREIRA Manuel F R, DELGADO Juan J, et al. Enhancement of the selectivity to dihydroxyacetone in glycerol oxidation using gold nanoparticles supported on carbon nanotubes[J]. Catalysis Communications, 2011, 16: 64-69. |
| 37 | PORTS Francesca, PRATI Laura. Selective oxidation of glycerol to sodium glycerate with gold-on-carbon catalyst: an insight into reaction selectivity[J]. Journal of Catalysis, 2004, 224(2): 397-403. |
| 38 | KETCHIE Willian C, MURAYAMA Mitsuhiro, DAVIS Robert J. Selective oxidation of glycerol over carbon-supported AuPd catalysts[J]. Journal of Catalysis, 2007, 250(2): 264-273. |
| 39 | RODRIGUES Elodie G, PEREIRA Manuel F R, CHEN Xiaowei, et al. Influence of activated carbon surface chemistry on the activity of Au/AC catalysts in glycerol oxidation[J]. Journal of Catalysis, 2011, 281(1): 119-127. |
| 40 | SUN Yanyan, LI Xuewen, WANG Jiangguo, et al. Carbon film encapsulated Pt NPs for selective oxidation of alcohols in acidic aqueous solution[J]. Applied Catalysis B: Environmental, 2017, 218(5): 538-544. |
| 41 | YANG Lihua, LI Xuewen, SUN Yangyang, et al. Selective oxidation of glycerol in base-free conditions over N-doped carbon film coated carbon supported Pt catalysts[J]. Catalysis Communications, 2017, 101: 107-110. |
| 42 | DIMITRATOS Nikolaos, VILLA Aaberto, PRATI Laura, et al. Effect of the preparation method of supported Au nanoparticles in the liquid phase oxidation of glycerol[J]. Applied Catalysis A: General, 2016, 514: 267-275. |
| 43 | ZHANG Mengyuan, SUN Yanyan, SHI Juanjuan, et al. Selective glycerol oxidation using platinum nanoparticles supported on multi-walled carbon nanotubes and nitrogen-doped graphene hybrid[J]. Chinese Journal of Catalysis, 2017, 38(3): 537-544. |
| 44 | LIANG Dan, GAO Jing, WANG Junhua, et al. Bimetallic Pt-Cu catalysts for glycerol oxidation with oxygen in a base-free aqueous solution[J]. Catalysis Communications, 2011, 12(12): 1059-1062. |
| 45 | TINCOCO Miguel, Susana FERNANDEZ-GARCIA, VILLA Alberto, et al. Selective oxidation of glycerol on morphology controlled ceria nanomaterials[J]. Catalysis Science & Technology, 2019, 9(9): 2328-2334. |
| 46 | PURUSHOTHAMAN Rajeesh Kumar Pazhavelikkakath, HAVEREN J VAN, VAN ES D S, et al. An efficient one pot conversion of glycerol to lactic acid using bimetallic gold-platinum catalysts on a nanocrystalline CeO2 support[J]. Applied Catalysis B: Environmental, 2014, 147: 92-100. |
| 47 | DIMITRATOS Nikolaos, LOPEZ-SANCHEZ Jose Antonic, ANTHONYKUTTY Jinto Manjaly, et al. Oxidation of glycerol using gold-palladium alloy-supported nanocrystals[J]. Physical Chemistry Chemical Physics, 2009, 11(25): 4952-4961. |
| 48 | XU Jilei, ZHANG Hongye, ZHAO Yanfei, et al. Selective oxidation of glycerol to lactic acid under acidic conditions using AuPd/TiO2 catalyst[J]. Green Chemistry, 2013, 15(6):1520-1525. |
| 49 | MUSIALSKA Karolina, FINOCCHIO Elisabetta, SOBCZAK Izabela, et al. Characterization of alumina- and niobia-supported gold catalysts used for oxidation of glycerol[J]. Applied Catalysis A: General, 2010, 384(1/2): 70-77. |
| 50 | PAN Yongning, WU Guandong, HE Yufei, et al. Identification of the Au/ZnO interface as the specific active site for the selective oxidation of the secondary alcohol group in glycerol[J]. Journal of Catalysis, 2019, 369: 222-232. |
| 51 | MENG Ye, ZOU Shihui, ZHOU Yuheng, et al. Activating molecular oxygen by Au/ZnO to selectively oxidize glycerol to dihydroxyacetone[J]. Catalysis Science & Technology, 2018, 8(10): 2524-2528. |
| 52 | LIU Shusen, SUN Keqiang, XU Boqing. Specific selectivity of Au-catalyzed oxidation of glycerol and other C3-polyols in water without the presence of a base[J]. ACS Catalysis, 2014, 4(7): 2226-2230. |
| 53 | 袁德玲. CuNiTi类水滑石衍生物富氧丙烯选择性催化还原NO的研究[D].大连:大连理工大学,2013. |
| YUAN Deling. CuNiTi hydrotalcite-derived catalysts for selective catalytic reduction of NO with C3H6 under lean-burn conditions[D]. Dalian: Dalian University of Technology, 2013. | |
| 54 | XU Chunli, SUN Jun, ZHAO Binbin, et al. On the study of KF/Zn(Al)O catalyst for biodiesel production from vegetable oil[J]. Applied Catalysis B: Environmental, 2010, 99(1/2): 111-117. |
| 55 | MURCIA-MASCAROS S, NAVARRO R M, GOMEZ-SAINERO L, et al. Oxidative methanol reforming reactions on CuZnAl catalysts derived from hydrotalcite-like precursors[J]. Journal of Catalysis, 2001, 198(2): 338-347. |
| 56 | CHOUDARY B M, KANTAM M L, RAHMAN A, et al. The first example of activation of molecular oxygen by nickel in Ni-Al hydrotalcite: a novel protocol for the selective oxidation of alcohols[J]. Angewandte Chemie: International Edition, 2001, 113(4): 785-788. |
| 57 | BARRAULT J, DEROUAULT A, COURTOIS G, et al. On the catalytic properties of mixed oxides obtained from the Cu-Mg-Al LDH precursors in the process of hydrogenation of the cinnamaldehyde[J]. Applied Catalysis A: General, 2004, 262(1): 43-51. |
| 58 | LI Landong, YU Jiejun, HAO Zhengping, et al. Novel Ru-Mg-Al-O catalyst derived from hydrotalcite-like compound for NO storage/decomposition/reduction[J]. The Journal of Physical Chemistry C, 2007, 111(28): 10552-10559. |
| 59 | KE Yihu, LI Xiaohua, LI Jifan, et al. Conversion of glycerol to dihydroxyacetone over Au catalysts on various supports[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(4): 1153-1162. |
| 60 | MOREAU Francois, BOND Geoffrey C, TAYLOR Adrian O. Gold on titania catalysts for the oxidation of carbon monoxide: control of pH during preparation with various gold contents[J]. Journal of Catalysis, 2005, 231(1): 105-114. |
| 61 | MOREAU Francois, BOND Geoffrey C. Gold on titania catalysts, influence of some physicochemical parameters on the activity and stability for the oxidation of carbon monoxide[J]. Applied Catalysis A: General, 2006, 302(1): 110-117. |
| 62 | YOU Kuenjiun, CHANG Chingtu, LIAW Biingjye, et al. Selective hydrogenation of α,β-unsaturated aldehydes over Au/MgxAlO hydrotalcite catalysts[J]. Applied Catalysis A: General, 2009, 361(1/2): 65-71. |
| 63 | PATZK􀆕 Ágnes, KUN Robert, HORNOK Viktória,et al. ZnAl-layer double hydroxides as photocatalysts for oxidation of phenol in aqueous solution[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 265(1/2/3): 64-72. |
| 64 | WOLF Anke, Ferdi SCHÜTH. A systematic study of the synthesis conditions for the preparation of highly active gold catalysts[J]. Applied Catalysis A: General, 2002, 226: 1-13. |
| 65 | LEPPELT R, SCHUMACHER B, PLZAK V, et al. Kinetics and mechanism of the low-temperature water-gas shift reaction on Au/CeO2 catalysts in an idealized reaction atmosphere[J]. Journal of Catalysis, 2006, 244(2): 137-152. |
| 66 | 毕博. 新型杂多酸类水滑石插层材料的制备和吸附、光催化性能研究[D]. 长春:东北师范大学,2012. |
| BI Bo. Study on preparation, adsorption and photocatalytic performance of polyoxometalated layered double hydroxides[D]. Changchun: Northeast Normal University, 2012. | |
| 67 | REICHLE Walter T. Catalytic reactions by thermally activated, synthetic, anionic clay minerals[J]. Journal of Catalysis, 1985, 94(2): 547-557. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [3] | WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410. |
| [4] | GAO Yufei, LU Jinfeng. Mechanism of heterogeneous catalytic ozone oxidation:A review [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 430-438. |
| [5] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [6] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [7] | WANG Fu'an. Consumption and emission reduction of the reactor of 300kt/a propylene oxide process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 213-218. |
| [8] | GENG Yuanze, ZHOU Junhu, ZHANG Tianyou, ZHU Xiaoyu, YANG Weijuan. Homogeneous/heterogeneous coupled combustion of heptane in a partially packed bed burner [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4514-4521. |
| [9] | GAO Yanjing. Analysis of international research trend of single-atom catalysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4667-4676. |
| [10] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [11] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [12] | LEI Wei, JIANG Weijia, WANG Yugao, HE Minghao, SHEN Jun. Synthesis of N,S co-doped coal-based carbon quantum dots by electrochemical oxidation and its application in Fe3+ detection [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4799-4807. |
| [13] | LI Dongze, ZHANG Xiang, TIAN Jian, HU Pan, YAO Jie, ZHU Lin, BU Changsheng, WANG Xinye. Research progress of NO x reduction by carbonaceous substances for denitration in cement kiln [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4882-4893. |
| [14] | WANG Chen, BAI Haoliang, KANG Xue. Performance study of high power UV-LED heat dissipation and nano-TiO2 photocatalytic acid red 26 coupling system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4905-4916. |
| [15] | ZHU Chuanqiang, RU Jinbo, SUN Tingting, XIE Xingwang, LI Changming, GAO Shiqiu. Characteristics of selective non-catalytic reduction of NO x with solid polymer denitration agent [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4939-4946. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||