Chemical Industry and Engineering Progress ›› 2020, Vol. 39 ›› Issue (4): 1292-1301.DOI: 10.16085/j.issn.1000-6613.2019-1155
• Energy processes and technology • Previous Articles Next Articles
Release of K during biomass combustion and pyrolysis: a review
Yang WANG1,2( ),Changqing DONG2(
),Changqing DONG2( )
)
- 1.Production Management and Environment Protection Department, China Huaneng Group Co. , Ltd, Beijing 100031, China
2.Renewable Energy School, North China Electric Power University, Beijing 102206, China
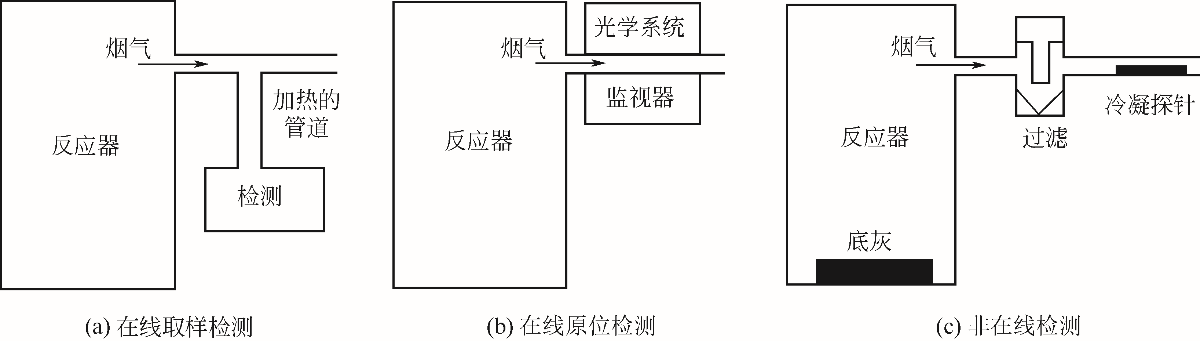
-
Received:2019-07-19Online:2020-04-28Published:2020-04-05 -
Contact:Changqing DONG
生物质燃烧和热解中钾的释放规律研究进展
- 1.中国华能集团有限公司生产环保部,北京 100031
2.华北电力大学可再生能源学院,北京 102206
-
通讯作者:董长青 -
作者简介:王洋(1988—),男,博士,研究方向为热能工程。E-mail:wyalex0805@126.com 。 -
基金资助:北京市自然科学基金(3172030)
CLC Number:
Cite this article
Yang WANG,Changqing DONG. Release of K during biomass combustion and pyrolysis: a review[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1292-1301.
王洋,董长青. 生物质燃烧和热解中钾的释放规律研究进展[J]. 化工进展, 2020, 39(4): 1292-1301.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2019-1155
| 生物质 | 质量分数/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | SO3 | P2O5 | |
| 红橡木 | 49.00 | 9.50 | 8.50 | 17.50 | 1.10 | 0.50 | 9.50 | — | 2.60 | 1.80 |
| 麦秸秆 | 55.32 | 1.88 | 0.73 | 6.14 | 1.06 | 1.71 | 25.60 | 0.08 | 4.40 | 1.26 |
| 榛子壳 | 33.70 | 3.10 | 3.80 | 15.40 | 7.90 | 1.30 | 30.40 | 0.10 | 1.10 | 3.20 |
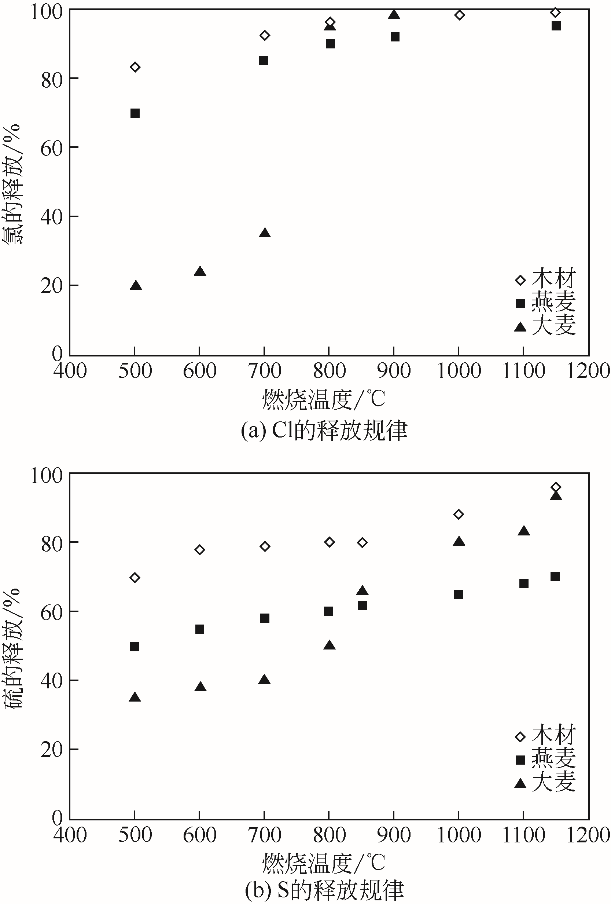
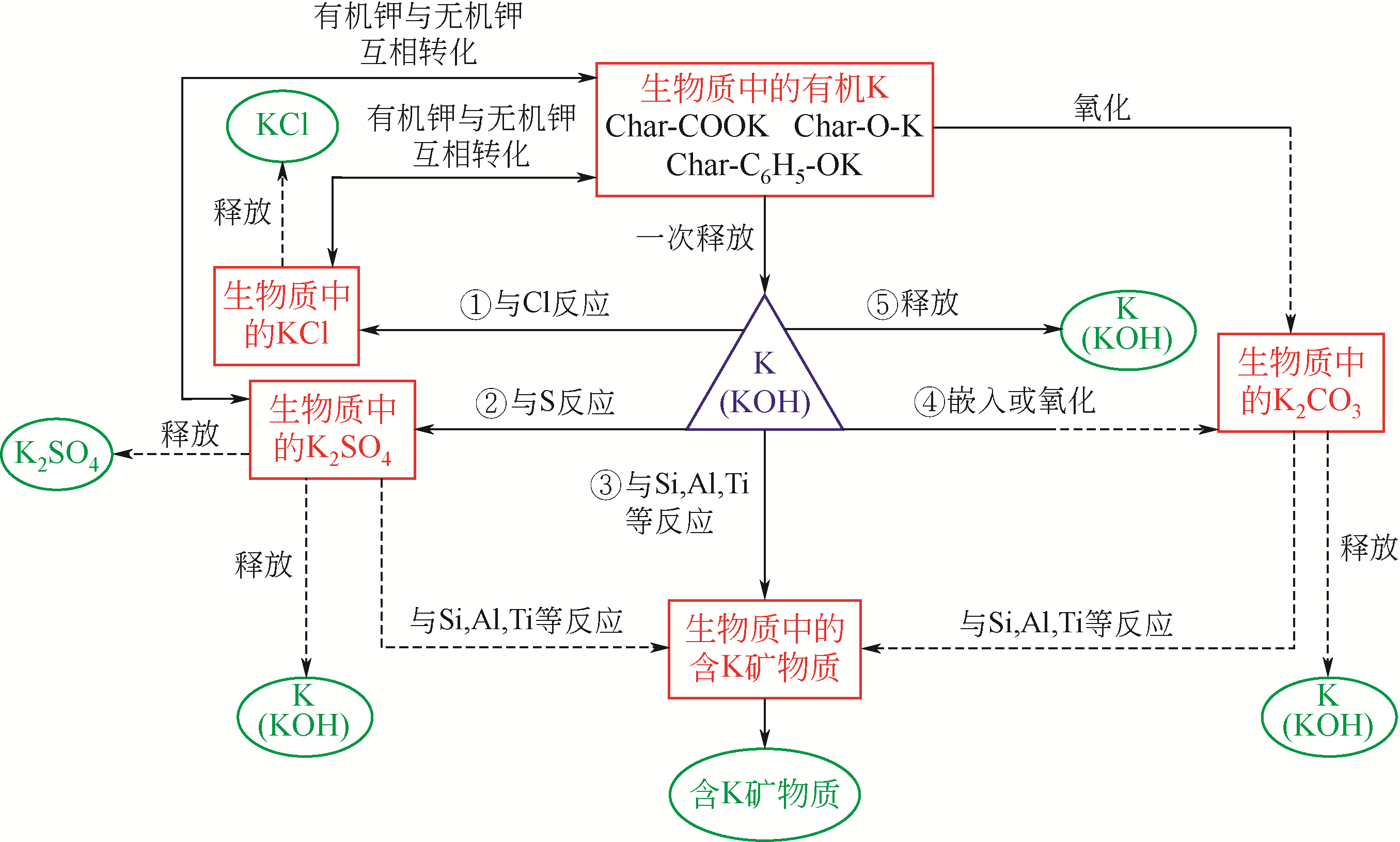
| 杏仁壳 | 23.50 | 2.70 | 2.80 | 10.50 | 5.20 | 1.60 | 48.50 | 0.10 | 0.80 | 4.50 |
| 生物质 | 质量分数/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | SO3 | P2O5 | |
| 红橡木 | 49.00 | 9.50 | 8.50 | 17.50 | 1.10 | 0.50 | 9.50 | — | 2.60 | 1.80 |
| 麦秸秆 | 55.32 | 1.88 | 0.73 | 6.14 | 1.06 | 1.71 | 25.60 | 0.08 | 4.40 | 1.26 |
| 榛子壳 | 33.70 | 3.10 | 3.80 | 15.40 | 7.90 | 1.30 | 30.40 | 0.10 | 1.10 | 3.20 |
| 杏仁壳 | 23.50 | 2.70 | 2.80 | 10.50 | 5.20 | 1.60 | 48.50 | 0.10 | 0.80 | 4.50 |
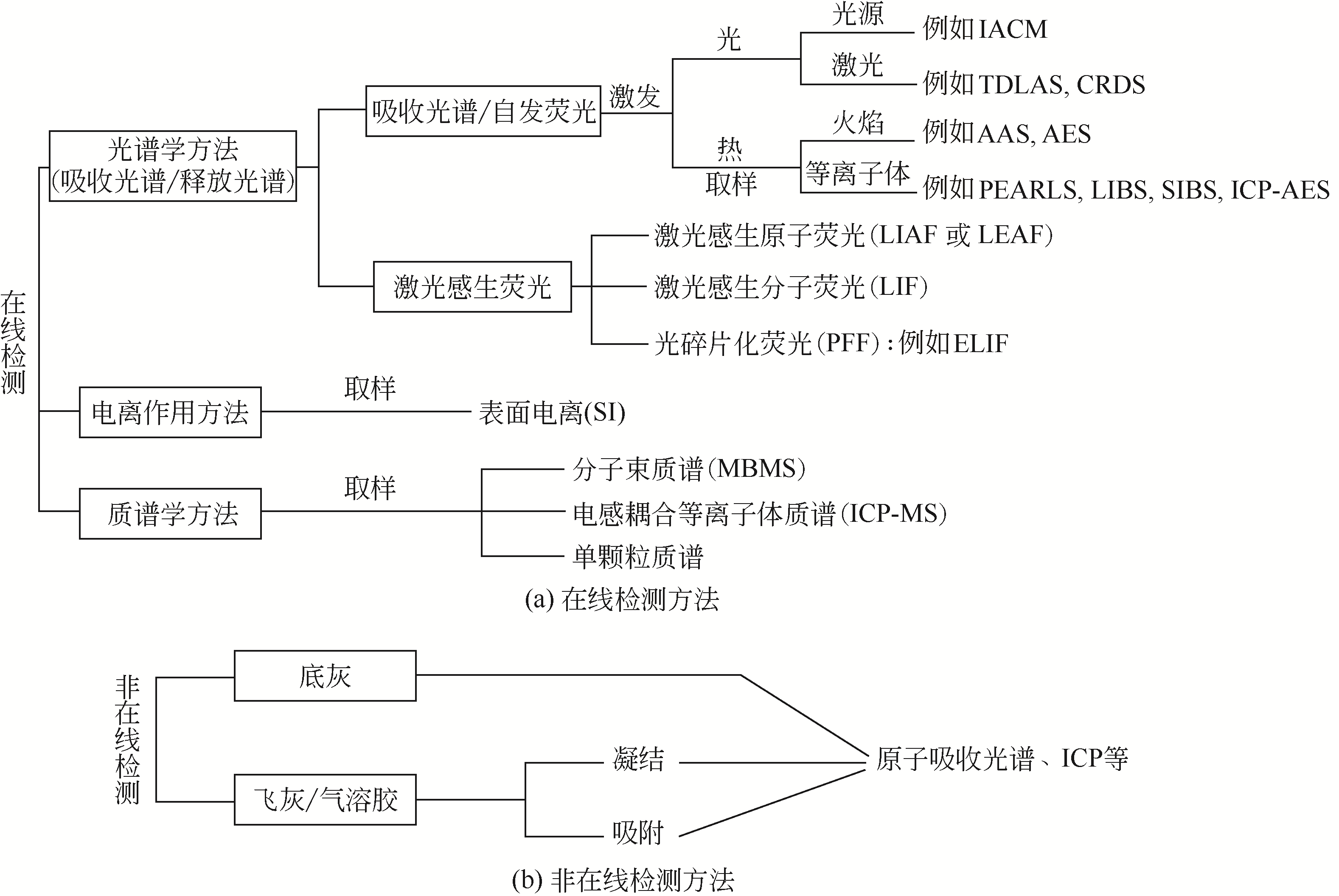
| 分析方法 | 原理 | 检测类型 | 检测的物质 | 检测精度 | 其他 |
|---|---|---|---|---|---|
| IACM | 光谱学 | 在线原位检测 | KC、NaCl | 1~50μg·g-1 | 检测碱金属氯化物 |
| TDLAS | 光谱学 | 在线原位检测 | 原子K、Na | >7.5ng·kg-1 | 仅气态 |
| PEARLS | 光谱学 | 在线取样检测 | 原子、Na | μg·kg-1 | 不区分相态 |
| LIBS | 光谱学 | 在线原位检测 | 原子K、Na | μg·g-1 | 不区分相态 |
| ELIF | 光谱学 | 在线原位检测 | 原子K、Na | μg·kg-1 | 仅气态 |
| SI | 电离作用 | 在线取样检测 | K+、Na+ | μg·kg-1 | 离子,不区分相态 |
| MBMS | 质谱学 | 在线取样检测 | KCl、KOH等 | μg·g-1 | 分子,不区分相态 |
| ICP-AES | 光谱学 | 非在线检测 | 原子K、Na | μg·kg-1 | 不区分相态 |
| 分析方法 | 原理 | 检测类型 | 检测的物质 | 检测精度 | 其他 |
|---|---|---|---|---|---|
| IACM | 光谱学 | 在线原位检测 | KC、NaCl | 1~50μg·g-1 | 检测碱金属氯化物 |
| TDLAS | 光谱学 | 在线原位检测 | 原子K、Na | >7.5ng·kg-1 | 仅气态 |
| PEARLS | 光谱学 | 在线取样检测 | 原子、Na | μg·kg-1 | 不区分相态 |
| LIBS | 光谱学 | 在线原位检测 | 原子K、Na | μg·g-1 | 不区分相态 |
| ELIF | 光谱学 | 在线原位检测 | 原子K、Na | μg·kg-1 | 仅气态 |
| SI | 电离作用 | 在线取样检测 | K+、Na+ | μg·kg-1 | 离子,不区分相态 |
| MBMS | 质谱学 | 在线取样检测 | KCl、KOH等 | μg·g-1 | 分子,不区分相态 |
| ICP-AES | 光谱学 | 非在线检测 | 原子K、Na | μg·kg-1 | 不区分相态 |
| 1 | 蒋剑春. 生物质能源应用研究现状与发展前景[J]. 林产化学与工业, 2002, 22(2): 75-80. |
| JIANG J C.Prospect on research and development ofbiomass energy utilization[J]. Chemistry and Industry of Forest Products, 2002, 22(2): 75-80. | |
| 2 | RAGLAND K W, AERTS D J, BAKER A J. Properties of wood for combustion analysis[J]. Bioresource Technology, 1991, 37(2):161-168. |
| 3 | MILLER R N. A geochemical study of the inorganic constituents in some low-rank coals[J]. Thesis Pennsylvania State Univ., 1978,13(1):146-149. |
| 4 | MILLER R N, GIVEN P H. The association of major, minor and trace inorganic elements with lignites. I. Experimental approach and study of a North Dakota lignite[J]. Geochimica et Cosmochimica Acta, 1986, 50(9): 2033-2043. |
| 5 | JENKINS B M, BAXTER L L, JR T R M, et al. Combustion properties of biomass[J]. Fuel Processing Technology, 1998, 54(1/2/3): 17-46. |
| 6 | ZEVENHOVEN O M. Ash-forming matter in biomass fuels[M]. Åbo/Turku, Finland: ÅboAkademi, 2001: 2-3. |
| 7 | WERKELIN J, SKRIFVARS B J, ZEVENHOVEN O M, et al. Chemical forms of ash-forming elements in woody biomass fuels[J]. Fuel, 2010, 89(2): 481-493. |
| 8 | FORSBERG C, BROSTRÖO M, BACKMAN R, et al. Principle, calibration, and application of the in situ alkali chloride monitor[J]. Review of Scientific Instruments, 2009, 80(2): 153-168. |
| 9 | HAYRINEN V, HERNBERG R, AHO M. Demonstration of plasma excited atomic resonance line spectroscopy for on-line measurement of alkali metals in a 20kW bubbling fluidized bed[J]. Fuel, 2004, 83(7): 791-797. |
| 10 | 韩雨佳, 杨燕梅, 许开龙, 等. 准东煤射流火焰碱金属析出在线测量研究[J]. 工程热物理学报, 2017, 38(6): 1351-1356. |
| HAN Y J, YANG Y M, XU K L, et al. On-line measurement of alkali metal released from the jet flames of Zhundong coal[J]. Journal of Engineering Thermophisics, 2017, 38(6): 1351-1356. | |
| 11 | 朱燕群, 钟厦, 何勇, 等. 准东煤化学处理后碱金属含量的激光测量研究[J]. 激光技术, 2017, 41(1): 101-105. |
| ZHU Y Q, ZHONG X, HE Y, et al. Measurement of alkali content in Zhundong coal after chemical fractionation treatment by LIBS method[J]. Laser Technology, 2017, 41(1): 101-105. | |
| 12 | 朱川. 高碱煤中碱金属含量测定及应用进展[J]. 洁净煤技术, 2018, 24(5): 20-25, 32. |
| ZHU C. Determination of alkali metal content in high alkali coal and its application[J]. Clean Coal Technology, 2018, 24(5): 20-25, 32. | |
| 13 | 杨光. 生物质燃烧过程中碱金属迁移研究[D]. 广州: 华南理工大学, 2012. |
| YANG G. Study on the alkali transformation behavior of straw combusiton[D]. Guangzhou: South China University of Technology, 2012. | |
| 14 | 田艳飞. 含碱金属燃烧火焰的发射光谱检测与分析[D]. 武汉: 华中科技大学, 2016. |
| TIAN Y F. Measurement and analysis of emission spectra from flames containing alkali metals[D]. Wuhan: Huazhong University of Science and Technology, 2016. | |
| 15 | 杨涛, 胡松, 向军, 等. 生物质热能利用过程中碱/碱土金属特性及检测技术研究进展[J]. 生物质化学工程, 2008, 42(6): 49-54. |
| YANG T, HU S, XIANG J, et al. Review on characteristics and detection technology of the alkali/alkaline earth metal during biomass thermal utilizaiton[J]. Biomass Chemical Engineering, 2008, 42(6): 49-54. | |
| 16 | GOTTWALD U, MONKHOUSE P. Single-port optical access for spectroscopic measurements in industrial flue gas ducts[J]. Applied Physics B, 1999, 69(2): 151-154. |
| 17 | MONKHOUSE P. On-line diagnostic methods for metal species in industrial process gas[J]. Progress in Energy and Combustion Science, 2002, 28(4): 331-381. |
| 18 | HELBLE J J, SRINIVASACHAR S, BONI A A, et al. Measurement and modeling of vapor-phase sodium chloride formed during pulverized coal combustion[J]. Combustion Science and Technology, 1992, 81(4-6): 193-205. |
| 19 | GOTTWALD U, MONKHOUSE P, WULGARIS N, et al. In-situ study of the effect of operating conditions and additives on alkali emissions in fluidised bed combustion[J]. Fuel Processing Technology, 2002, 75(3): 215-226. |
| 20 | FRENCH R J, DAYTON D C, MILNE T A. The direct observation of alkali vapor species in biomass combustion and gasification[R]. Golden, CO (United States): National Renewable Energy Lab., 1994-01-20. |
| 21 | DAYTON D C, FRENCH R J, MILNE T A. Direct observation of alkali vapor release during biomass combustion and gasification. 1. Aapplication of molecular beam/mass spectrometry to switchgrass combustion[J]. Energy & Fuels, 1995, 9(5): 855-865. |
| 22 | BLASING M, MULLER M. Mass spectrometric investigations on the release of inorganic species during gasification and combustion of Rhenish lignite[J]. Fuel, 2010, 89(9): 2417-2424. |
| 23 | ZARCHY A S. An alkali metal detector for use in fluidized bed combustor effluent streams[C]//5th Symp. on Instrumentation and Control for Fossil Energy Processes, USA,1981. |
| 24 | DAVIDSSON K O, STOJKOVA B J, PETTERSSON J B C. Alkali emission from birchwood particles during rapid pyrolysis[J]. Energy & Fuels, 2002, 16(5): 1033-1039. |
| 25 | DAVIDSSON K O, ENGVALL K, HAGSTRÖM M, et al. A surface ionization instrument for on-line measurements of alkali metal components in combustion: instrument description and applications[J]. Energy &Fuels, 2002, 16(6): 1369-1377. |
| 26 | LIAW S B, WU H. Leaching characteristics of organic and inorganic matter from biomass by water: differences between batch and semi-continuous operations[J]. Industrial & Engineering Chemistry Research, 2013, 52(11): 4280-4289. |
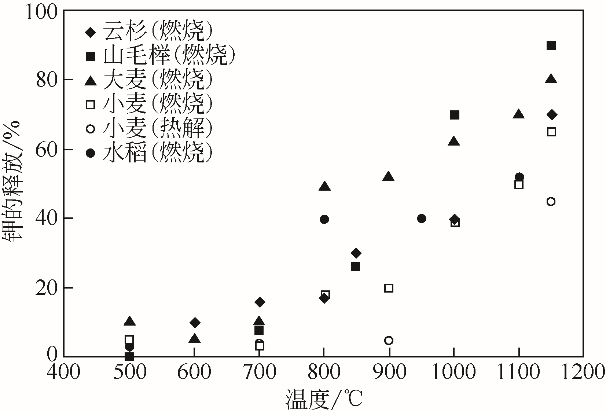
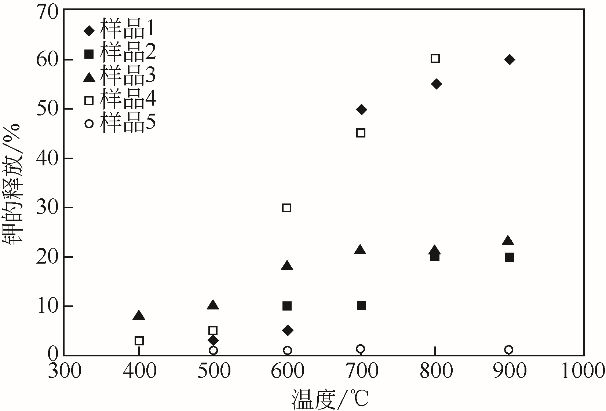
| 27 | ZHANG Z H, SONG Q, ALWAHABI Z T, et al. Temporal release of potassium from pinewood particles during combustion[J]. Combustion and Flame, 2015, 162(2): 496-505. |
| 28 | FATEHI H, HESAMEDDIN Y, WANG Z, et al. LIBS measurements and numerical studies of potassium release during biomass gasification[J]. Proceedings of the Combustion Institute, 2015, 35(2): 2389-2396. |
| 29 | FATEHI H, LI Z S, BAI X S, et al. Modeling of alkali metal release during biomass pyrolysis[J]. Proceedings of the Combustion Institute, 2017, 36(2): 2243-2251. |
| 30 | HUPAM M. Ash-related issues in fluidized-bed combustion of biomasses: recent research highlights[J]. Energy & Fuels, 2011, 26(1): 4-14. |
| 31 | JENSEN P A, FRANDSEN F J, DAM-JOHANSEN K, et al. Experimental investigation of the transformation and release to gas phase of potassium and chlorine during straw pyrolysis[J]. Energy & Fuels, 2000, 14(6): 1280-1285. |
| 32 | KNUDSEN J N, JENSEN P A, DAM-JOHANSEN K. Transformation and release to the gas phase of Cl, K, and S during combustion of annual biomass[J]. Energy & Fuels, 2004, 18(5): 1385-1399. |
| 33 | KNUDSEN J N, JENSEN P A, LIN W, et al. Secondary capture of chlorine and sulfur during thermal conversion of biomass[J]. Energy & Fuels, 2005, 19(2): 606-617. |
| 34 | VAN-LITH S C, ALONSO-RAMÍREZ V, JENSEN P A, et al. Release to the gas phase of inorganic elements during wood combustion. part 1: Development and evaluation of quantification methods[J]. Energy &Fuels, 2006, 20(3): 964-978. |
| 35 | VAN-LITH S C, JENSEN P A, FRANDSEN F J, et al. Release to the gas phase of inorganic elements during wood combustion. part 2: Influence of fuel composition[J]. Energy & Fuels, 2008, 22(3): 1598-1609. |
| 36 | OKUNO T, SONOYAMA N, HAYASHI J, et al. Primary release of alkali and alkaline earth metallic species during the pyrolysis of pulverized biomass[J]. Energy & Fuels, 2005, 19(5): 2164-2171. |
| 37 | KEOWN D M, FAVAS G, HAYASHI J, et al. Volatilisation of alkali and alkaline earth metallic species during the pyrolysis of biomass: differences between sugar cane bagasse and cane trash[J]. Bioresource Technology, 2005, 96(14): 1570-1577. |
| 38 | ZINTL F, STRÖMBERG B, BJÖRKMAN E. Release of chlorine from biomass at gasification conditions[C]//Biomass for energy and Industry, 10th European Conference and Technology Exhibition, Germany, 1998, 1608. |
| 39 | BRIDGWATER A V. Progress in thermochemical biomass conversion[M]. Britain: Blackwell Science, 2001: 1234-1245. |
| 40 | HAMILTON J T G, MCROBERTSS W C, KEPPLER F, et al. Chloride methylation by plant pectin: an efficient environmentally significant process[J]. Science, 2003, 301(5630): 206-209. |
| 41 | SALEH S,FLENSBORG J P, SHOULAIFAR T K, et al. Release of chlorine and sulfur during biomass torrefaction and pyrolysis[J]. Energy & Fuels, 2014, 28(6): 3738-3746. |
| 42 | SAILAUKHANULY Y,SÁROSSY Z, CARLSEN L, et al. Mechanistic aspects of the nucleophilic substitution of pectin on the formation of chloromethane[J]. Chemosphere, 2014, 111: 575-579. |
| 43 | CZÉGÉNY Z, JAKAB E, BOZI J, et al. Pyrolysis of wood-PVC mixtures. formation of chloromethane from lignocellulosic materials in the presence of PVC[J]. Journal of Analytical and Applied Pyrolysis, 2015, 113: 123-132. |
| 44 | DAYTON D C, JENKINS B M, TURN S Q, et al. Release of inorganic constituents from leached biomass during thermal conversion[J]. Energy & Fuels, 1999, 13(4): 860-870. |
| 45 | WIGMANS T, ELFRING R, MOULIJN J A. On the mechanism of the potassium carbonate catalysed gasification of activated carbon: the influence of the catalyst concentration on the reactivity and selectivity at low steam pressures[J]. Carbon, 1983, 21(1): 1-12. |
| 46 | HASHIMOTO K, MIURA K, XU J J, et al. Relation between the gasification rate of carbons supporting alkali metal salts and the amount of oxygen trapped by the metal[J]. Fuel, 1986, 65(4): 489-494. |
| 47 | LI X, LI C Z. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. part VIII. Catalysis and changes in char structure during gasification in steam[J]. Fuel, 2006, 85(10): 1518-1525. |
| 48 | WU H, QUYN D M, LI C Z. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. part III. The importance of the interactions between volatiles and char at high temperature[J]. Fuel, 2002, 81(8): 1033-1039. |
| 49 | LI X, WU H, HAYASHI J, et al. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal. part VI. Further investigation into the effects of volatile-char interactions[J]. Fuel, 2004, 83(10): 1273-1279. |
| 50 | JOHANSEN J M, JAKOBSEN J G, FRANDSEN F J, et al. Release of K, Cl, and S during pyrolysis and combustion of high-chlorine biomass[J]. Energy & Fuels, 2011, 25(11): 4961-4971. |
| 51 | OLSSON J G, JÄGLID U, PETTERSSON J B C, et al. Alkali metal emission during pyrolysis of biomass[J]. Energy & Fuels, 1997, 11(4): 779-784. |
| 52 | DAYTON D C, MILNE T A. Applications of advanced technology to ash-related problems in boilers[M]. US: Springer US, 1996: 161-185. |
| 53 | MISRA M K, RAGLAND K W, BAKER A J. Wood ash composition as a function of furnace temperature[J]. Biomass and Bioenergy, 1993, 4(2): 103-116. |
| [1] | LI Ning, LI Jinke, DONG Jinshan. Research and development of porous medium burner in ethylene cracking furnace [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 73-83. |
| [2] | GENG Yuanze, ZHOU Junhu, ZHANG Tianyou, ZHU Xiaoyu, YANG Weijuan. Homogeneous/heterogeneous coupled combustion of heptane in a partially packed bed burner [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4514-4521. |
| [3] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [4] | LI Zhiyuan, HUANG Yaji, ZHAO Jiaqi, YU Mengzhu, ZHU Zhicheng, CHENG Haoqiang, SHI Hao, WANG Sheng. Characterization of heavy metals during co-pyrolysis of sludge with PVC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4947-4956. |
| [5] | WANG Shuaiqing, YANG Siwen, LI Na, SUN Zhanying, AN Haoran. Research progress on element doped biomass carbon materials for electrochemical energy storage [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4296-4306. |
| [6] | LIU Shuqiong, WU Fangfang, LIU Ruilai, XU Zhenyi. Preparation and characterization of a novel polylactic acid/chitosan/graphene oxide/aspirin drug-loaded biomimetic composite scaffold [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4362-4371. |
| [7] | WU Ya, ZHAO Dan, FANG Rongmiao, LI Jingyao, CHANG Nana, DU Chunbao, WANG Wenzhen, SHI Jun. Research progress on highly efficient demulsifiers for complex crude oil emulsions and their applications [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4398-4413. |
| [8] | ZHENG Mengqi, WANG Chengye, WANG Yan, WANG Wei, YUAN Shoujun, HU Zhenhu, HE Chunhua, WANG Jie, MEI Hong. Application and prospect of algal-bacterial symbiosis technology in zero liquid discharge of industrial wastewater [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4424-4431. |
| [9] | LI Haidong, YANG Yuankun, GUO Shushu, WANG Benjin, YUE Tingting, FU Kaibin, WANG Zhe, HE Shouqin, YAO Jun, CHEN Shu. Effect of carbonization and calcination temperature on As(Ⅲ) removal performance of plant-based Fe-C microelectrolytic materials [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3652-3663. |
| [10] | GUAN Hongling, YANG Hui, JING Hongquan, LIU Yuqiong, GU Shouyu, WANG Haobin, HOU Cuihong. Lignin-based controlled release materials and application in drug delivery and fertilizer controlled-release [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3695-3707. |
| [11] | YANG Ziyu, ZHU Ling, WANG Wenlong, YU Chaofan, SANG Yimin. Research and application progress of smoldering combustion technology for oily sludge [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3760-3769. |
| [12] | YAO Liming, WANG Yazhuo, FAN Honggang, GU Qing, YUAN Haoran, CHEN Yong. Treatment status of kitchen waste and its research progress of pyrolysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3791-3801. |
| [13] | ZHANG Shan, ZHONG Zhaoping, YANG Yuxuan, DU Haoran, LI Qian. Enrichment of heavy metals in pyrolysis of municipal solid waste by phosphate modified kaolin [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3893-3903. |
| [14] | LI Dongxian, WANG Jia, JIANG Jianchun. Producing biofuels from soapstock via pyrolysis and subsequent catalytic vapor-phase hydrotreating process [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2874-2883. |
| [15] | WANG Baowen, LIU Tongqing, ZHANG Gang, LI Weiguang, LIN Deshun, WANG Mengjia, MA Jingjing. Reaction characteristics of CuFe2O4 modified desulfurization slag oxygen carrier with lignite [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2884-2894. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||