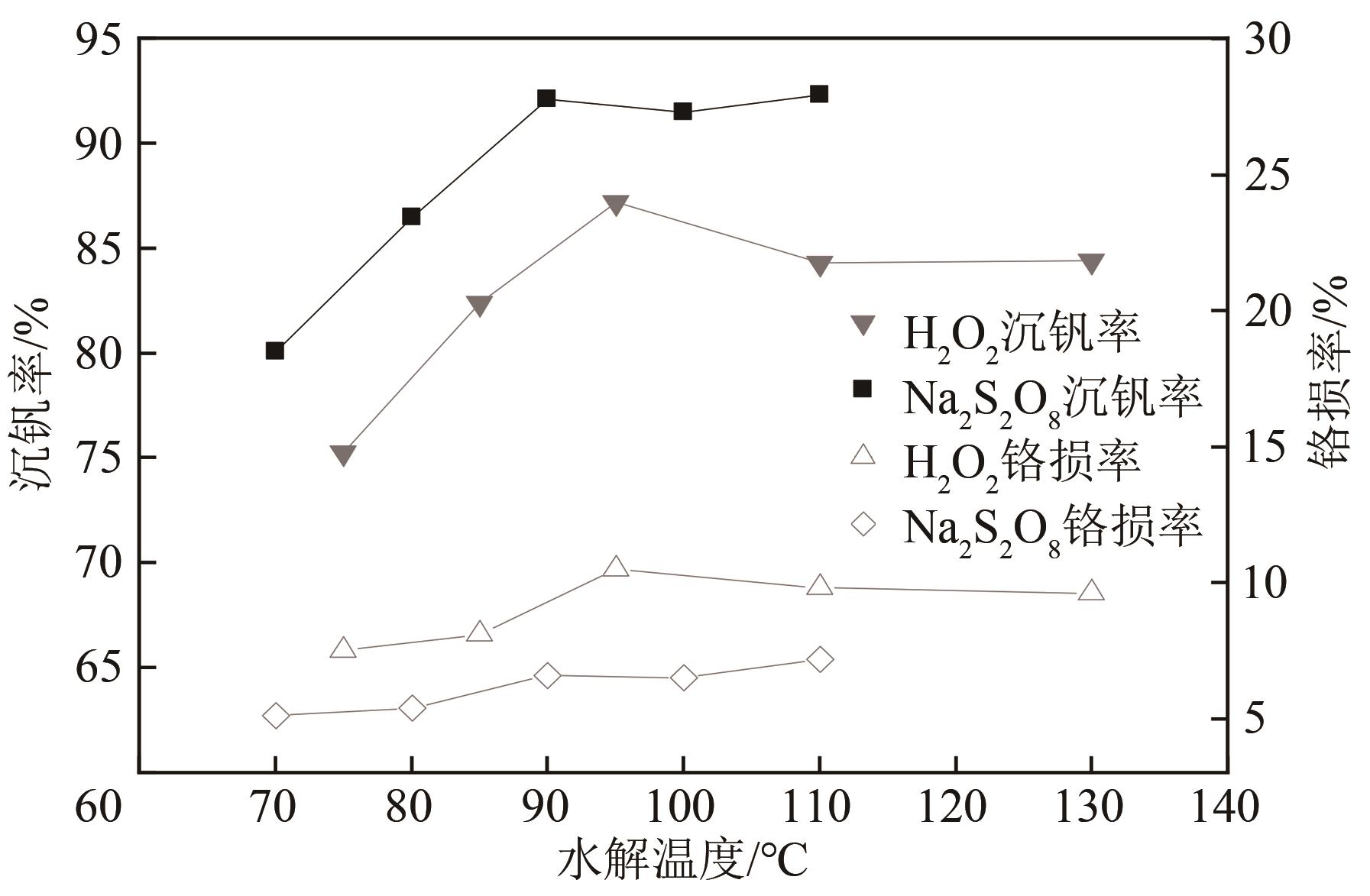
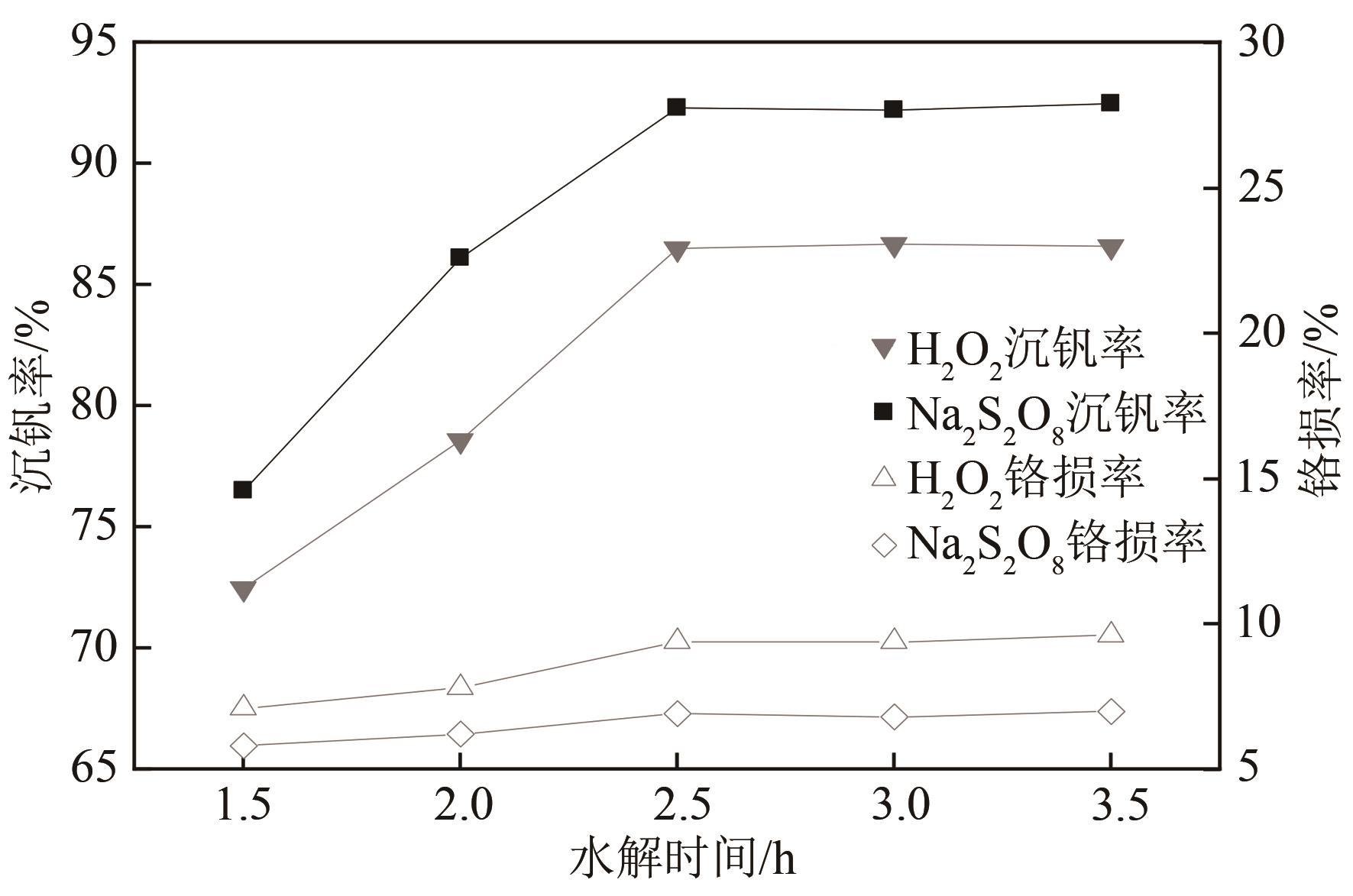
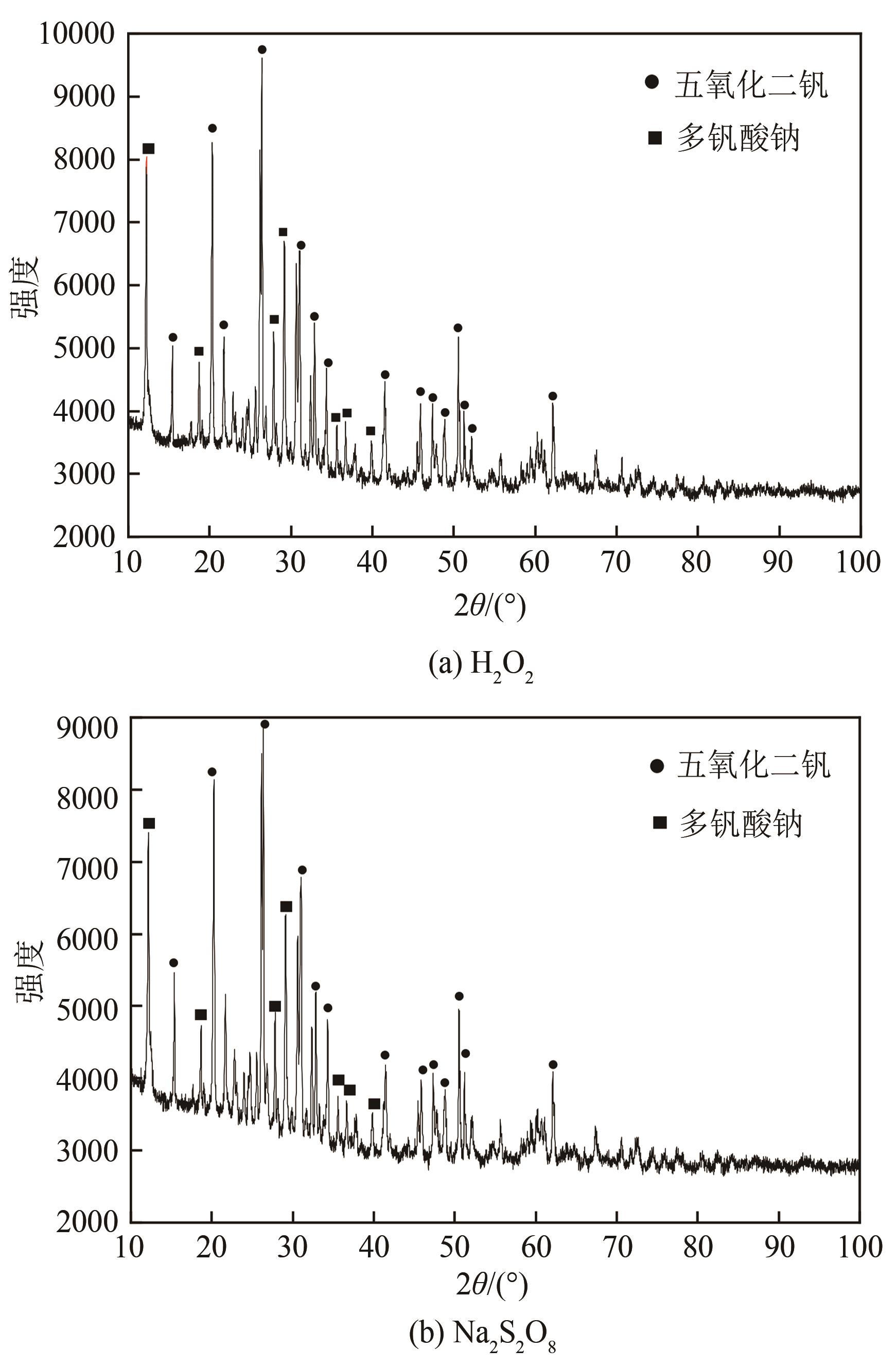
| 1 |
马闯, 高峻峰, 黄振宇, 等. 从含钒铬泥中提取V、Cr的工艺研究[J]. 稀有金属与硬质合金, 2016, 44(3): 17-20.
|
|
MA Chuang, GAO Junfeng, HUANG Zhenyu, et al. Technological study on extraction of vanadium and chromium from vanadium-bearing chromium residue[J]. Rare Metals and Cemented Carbides, 2016, 44(3): 17-20.
|
| 2 |
杨静翎, 金鑫. 酸浸法提钒新工艺的研究[J]. 北京化工大学学报, 2007, 34(3): 254-257.
|
|
YANG Jingling, JIN Xin. Study on new technology of vanadium extraction by acid leaching[J]. Journal of Beijing University of Chemical Technology, 2007, 34(3): 254-257.
|
| 3 |
PENG Xuefeng, ZHANG Yang, FAN Bingqiang, et al. Complexation separation for vanadium and chromium by dithiocarbamate and its application in treatment of chromium-vanadium-bearing slag[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(11): 2400-2410.
|
| 4 |
蒋霖. 钒铬溶液中钒铬提取及分离工艺研究进展[J]. 钢铁钒钛, 2014, 35(6): 52-59.
|
|
JIANG Lin. Research progress of the separation process of vanadium and chromium from solution containing vanadium and chromium[J]. Iron Steel Vanadium Titanium, 2014, 35(6): 52-59.
|
| 5 |
高官金. 钒铬液水热水解法提钒技术研究[J]. 钢铁钒钛, 2017, 38(5): 15-19.
|
|
GAO Guanjin. Vanadium extraction from vanadium-chromium solution by hydrothermal-hydrolysis precipitation[J]. Iron Steel Vanadium Titanium, 2017, 38(5): 15-19.
|
| 6 |
谭其尤, 陈波, 张裕书, 等. 攀西地区钒钛磁铁矿资源特点与综合回收利用现状[J]. 矿产综合利用, 2011(6): 6-10.
|
|
TAN Qiyou, CHEN Bo, ZHANG Yushu, et al. Characteristics and current situation of comprehensive utilization of vanadium titano-magnetite resources in Panxi region[J]. Multipurpose Utilization of Mineral Resources, 2011(6): 6-10.
|
| 7 |
李兰杰, 陈东辉, 白瑞国, 等. 钒渣中钒铬提取技术研究进展[J]. 矿产综合利用, 2013(2): 7-11.
|
|
LI Lanjie, CHEN Donghui, BAI Ruiguo, et al. Research progress of extraction technology for vanadium & chromium from vanadium slags[J]. Multipurpose Utilization of Mineral Resources, 2013(2): 7-11.
|
| 8 |
蒋霖, 付自碧, 伍珍秀. 酸性铵盐沉钒在钒铬溶液中的应用研究[J]. 钢铁钒钛, 2019, 40(3): 21-25.
|
|
JIANG Lin, FU Zibi, WU Zhenxiu. Research on application of acidic precipitation of vanadium with ammonium salt in the solution containing vanadium and chromium[J]. Iron Steel Vanadium Titanium, 2019, 40(3): 21-25.
|
| 9 |
李国良. 用沉淀法自钒铬溶液中分离和回收钒铬[J]. 钢铁钒钛, 1981, 2(3): 41-49.
|
|
LI Guoliang. Separation and recovery of vanadium and chromium from vanadium chromium solution by precipitation method[J]. Iron Steel Vanadium Titanium, 1981, 2(3): 41-49.
|
| 10 |
FAN Yeye, WANG Xuewen, WANG Mingyu. Separation and recovery of chromium and vanadium from vanadium-containing chromate solution by ion exchange[J]. Hydrometallurgy, 2013, 136: 31-35.
|
| 11 |
SHANG Guanghao, ZHANG Guiqing, GAO Congjie, et al. A novel nanofiltration process for the recovery of vanadium from acid leach solution[J]. Hydrometallurgy, 2014, 142: 94-97.
|
| 12 |
HU Qiaoyu, PAUDYAL Hari, ZHAO Junmei, et al. Adsorptive recovery of vanadium(Ⅴ) from chromium(Ⅵ)-containing effluent by Zr(Ⅳ)-loaded orange juice residue[J]. Chemical Engineering Journal, 2014, 248: 79-88.
|
| 13 |
彭雪枫. 钒铬泥资源化利用中铬/钒/铁络合深度分离机理研究[D]. 北京: 中国科学院大学(中国科学院过程工程研究所), 2019.
|
|
PENG Xuefeng. Mechanism of complexation deep separation of chromium, vanadium and iron in V-Cr-bearing reducing slag resource utilization technology[D]. Beijing: Institute of Process Engineering, Chinese Academy of Sciences, 2019.
|
| 14 |
罗卫, 强敏, 唐雪萍, 等. 两种纳米结构五氧化二钒的合成与表征[J]. 工业安全与环保, 2013, 39(2): 94-96.
|
|
LUO Wei, QIANG Min, TANG Xueping, et al. Synthesis and characterization of two kinds of nanostructure of vanadium pentoxide[J]. Industrial Safety and Environmental Protection, 2013, 39(2): 94-96.
|
 ), 韦林森1, 申长帅2(
), 韦林森1, 申长帅2( ), 李千文1, 汪超1, 范兵强3, 张贺东2, 张洋3(
), 李千文1, 汪超1, 范兵强3, 张贺东2, 张洋3( )
)
 ), WEI Linsen1, SHEN Changshuai2(
), WEI Linsen1, SHEN Changshuai2( ), LI Qianwen1, WANG Chao1, FAN Bingqiang3, ZHANG Hedong2, ZHANG Yang3(
), LI Qianwen1, WANG Chao1, FAN Bingqiang3, ZHANG Hedong2, ZHANG Yang3( )
)