化工进展 ›› 2023, Vol. 42 ›› Issue (4): 2003-2012.DOI: 10.16085/j.issn.1000-6613.2022-1025
脂质体递送系统功能结构设计与应用研究进展
- 1.清华大学机械工程系,北京 100084
2.新希望集团有限公司,四川 成都 610063
-
收稿日期:2022-06-02修回日期:2022-08-19出版日期:2023-04-25发布日期:2023-05-08 -
通讯作者:周明 -
作者简介:李建雄(1977—),男,博士,研究方向为先进成形制造。E-mail:lijx@newhope.cn。 -
基金资助:四川省国际科技创新合作(2020YFH0177)
Research progress on functional structure design and application of liposome delivery system
LI Jianxiong1,2( ), GENG Shuang2, HU Shujian1, ZHOU Ming1(
), GENG Shuang2, HU Shujian1, ZHOU Ming1( )
)
- 1.Department of Mechanical Engineering, Tsinghua University, Beijing 100084, China
2.New Hope Group Co. , Ltd. , Chengdu 610063, Sichuan, China
-
Received:2022-06-02Revised:2022-08-19Online:2023-04-25Published:2023-05-08 -
Contact:ZHOU Ming
摘要:
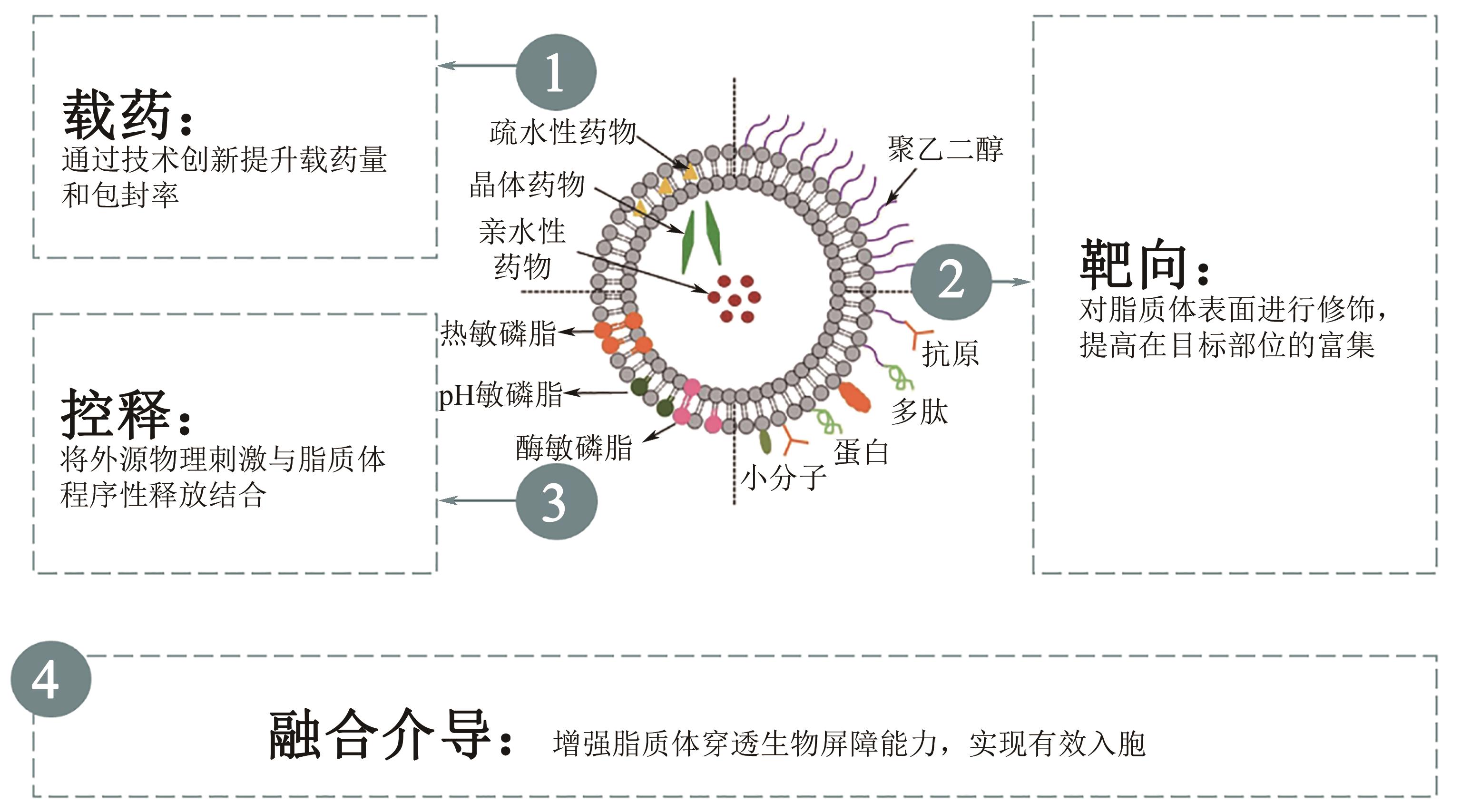
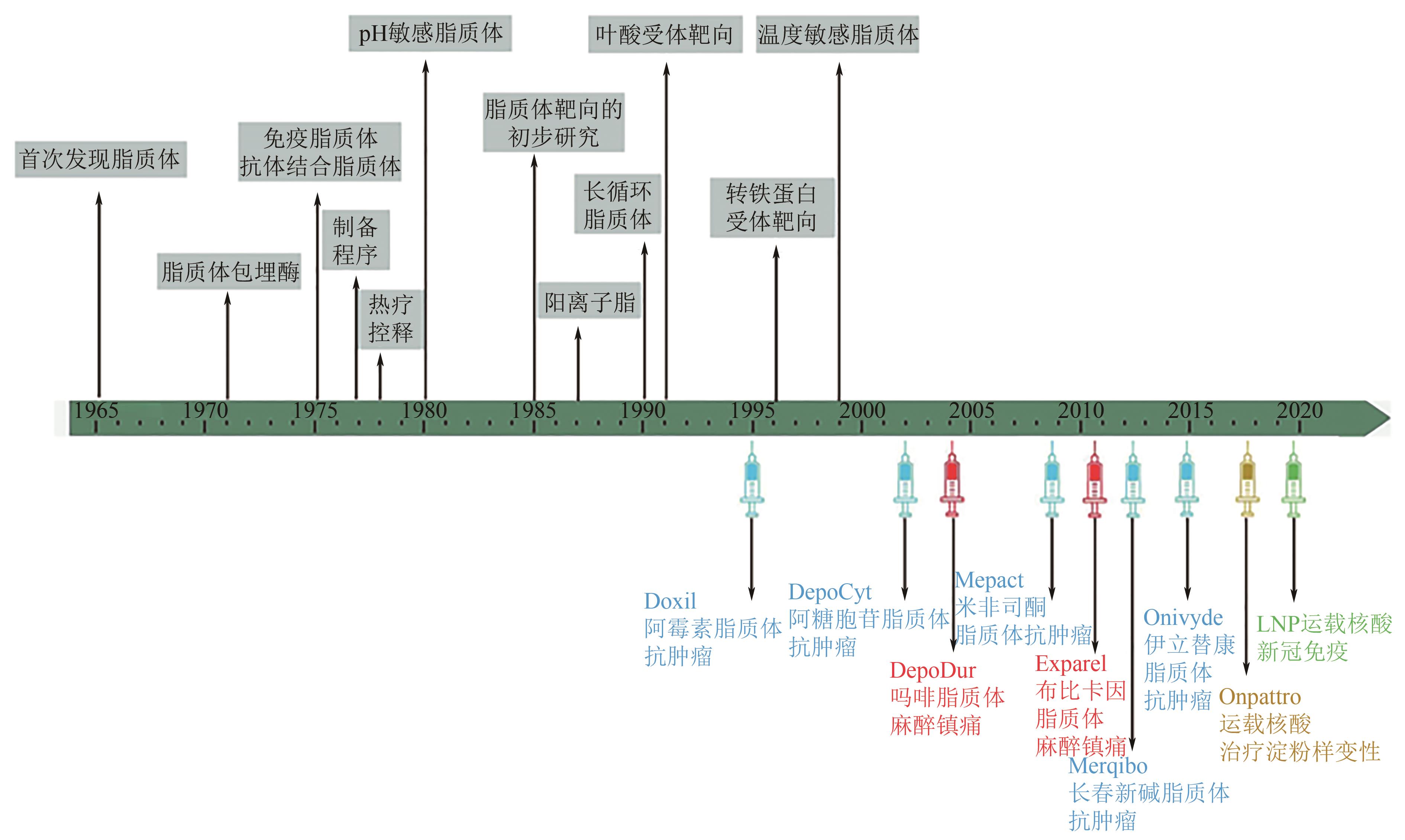
脂质体是人工制备的中空微球,由胆固醇、磷脂及其衍生物组装而成。得益于多样化的磷脂组分、粒径和中空球形结构,综合一系列电学、力学、材料学、表界面学特点,脂质体拥有优异的生物相容性和代谢属性,并具有靶向性和微环境响应能力,在药物递送、疾病诊断、高端日化及功能食品开发等领域有广泛研究。脂质体最成功的应用方式是作为纳米生物医药递送系统,研究成果和成熟产品不断涌现。基于此,本文从脂质体分子组成、空间特性、电学特性、表界面力学等结构特征出发,明确脂质体功能化设计核心要点,即创新制备技术以提升载药量和包封率,通过表面修饰以加强目标部位富集,将外源物理刺激与结构设计结合以实现程序释药,增强穿膜能力以完成有效入胞。结合最新研究,本文对脂质体负载药物、核酸、疫苗等成分的临床应用情况进行总结。
中图分类号:
引用本文
李建雄, 耿爽, 胡树坚, 周明. 脂质体递送系统功能结构设计与应用研究进展[J]. 化工进展, 2023, 42(4): 2003-2012.
LI Jianxiong, GENG Shuang, HU Shujian, ZHOU Ming. Research progress on functional structure design and application of liposome delivery system[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2003-2012.
| 治疗领域 | 主要候选药品 | 适应证 | 公司 | 临床/上市 | 临床/上市时间 |
|---|---|---|---|---|---|
| 抗肿瘤 | 多柔比星 | 多种实体瘤 | Alza | 上市 | 1995-11 |
| 乳腺癌 | Elan Pharmaceuticals | 上市 | 2000-07 | ||
| 卡波西肉瘤 | 上海复旦张江 | 上市 | 2005-12 | ||
| 多种实体瘤 | 石药中奇 | 上市 | 2008-12 | ||
| 卡波西肉瘤 | 常州金源 | 上市 | 2011-02 | ||
| 多种实体瘤 | Celsion | 临床Ⅲ期 | 2015-12 | ||
| 紫杉醇 | 多种实体痛 | 绿叶思科 | 上市 | 2007-03 | |
| 乳腺癌 | 南京泛太 | 临床Ⅱ期 | 2015-05 | ||
| 多种实体瘤 | 石药集团 | 临床Ⅲ期 | 2017-07 | ||
| 多种实体瘤 | 上海天汇 | 临床Ⅰ期 | 2006-09 | ||
| 伊立替康 | 转移性上皮膀胱癌 | Ipsen | 上市 | 2015-10 | |
| 胰腺癌 | 恒瑞医药 | 临床Ⅲ期 | 2013-09 | ||
| 多种实体瘤 | 齐鲁制药 | 临床Ⅰ期 | 2016-08 | ||
| 晚期实体瘤 | 绿叶制药 | 临床Ⅰ期 | 2017-03 | ||
| 晚期实体瘤 | 科伦药业 | 临床Ⅰ期 | 2017-03 | ||
| 晚期大肠癌 | 上海景峰 | 临床Ⅰ期 | 2017-09 | ||
| 柔红霉素 | 卡波西肉瘤 | NeXstar | 上市 | 1996-04 | |
| 阿糖胞苷 | 恶性淋巴癌 | Pacira | 上市 | 1999-04 | |
| 阿糖胞苷/柔红霉素 | 急性粒细胞白血病 | Jazz Pharmaceuticals | 上市 | 2018-08 | |
| 长春新碱 | 急性髓细胞性白血病,黑色素瘤 | Spectrum | 上市 | 2012-08 | |
| 急性髓细胞性白血病 | 上海复旦张江 | 上市 | 2009-03 | ||
| 和厚朴酚 | 晚期非小细胞肺癌 | 成都金瑞 | 临床Ⅰ期 | 2016-05 | |
| 抗感染 | 两霉素B | 真菌感柒 | NcXstar | 上市 | 1990-08 |
| 真菌感染 | 上海上药新亚 | 上市 | 2003-08 | ||
| 真菌感染 | 北京泰德 | 临床Ⅰ期 | 2015-08 | ||
| 真菌感染 | 石药集团 | 临床Ⅲ期 | 2021-11 | ||
| 真菌感染 | 台湾东洋 | 临床Ⅰ期 | 2017-05 | ||
| 麻醉 | 硫酸吗啡 | 术后疼痛 | Pacira | 上市 | 2004-05 |
| 布尔卡因 | 术后疼痛 | Pacira | 上市 | 2011-10 | |
| 心脑血管 | 前列地尔 | 下肢动脉硬化闭塞症 | 石药集团 | 临床Ⅱ期 | 2019-09 |
表1 全球范围内获批上市和国内获批临床研究的脂质体
| 治疗领域 | 主要候选药品 | 适应证 | 公司 | 临床/上市 | 临床/上市时间 |
|---|---|---|---|---|---|
| 抗肿瘤 | 多柔比星 | 多种实体瘤 | Alza | 上市 | 1995-11 |
| 乳腺癌 | Elan Pharmaceuticals | 上市 | 2000-07 | ||
| 卡波西肉瘤 | 上海复旦张江 | 上市 | 2005-12 | ||
| 多种实体瘤 | 石药中奇 | 上市 | 2008-12 | ||
| 卡波西肉瘤 | 常州金源 | 上市 | 2011-02 | ||
| 多种实体瘤 | Celsion | 临床Ⅲ期 | 2015-12 | ||
| 紫杉醇 | 多种实体痛 | 绿叶思科 | 上市 | 2007-03 | |
| 乳腺癌 | 南京泛太 | 临床Ⅱ期 | 2015-05 | ||
| 多种实体瘤 | 石药集团 | 临床Ⅲ期 | 2017-07 | ||
| 多种实体瘤 | 上海天汇 | 临床Ⅰ期 | 2006-09 | ||
| 伊立替康 | 转移性上皮膀胱癌 | Ipsen | 上市 | 2015-10 | |
| 胰腺癌 | 恒瑞医药 | 临床Ⅲ期 | 2013-09 | ||
| 多种实体瘤 | 齐鲁制药 | 临床Ⅰ期 | 2016-08 | ||
| 晚期实体瘤 | 绿叶制药 | 临床Ⅰ期 | 2017-03 | ||
| 晚期实体瘤 | 科伦药业 | 临床Ⅰ期 | 2017-03 | ||
| 晚期大肠癌 | 上海景峰 | 临床Ⅰ期 | 2017-09 | ||
| 柔红霉素 | 卡波西肉瘤 | NeXstar | 上市 | 1996-04 | |
| 阿糖胞苷 | 恶性淋巴癌 | Pacira | 上市 | 1999-04 | |
| 阿糖胞苷/柔红霉素 | 急性粒细胞白血病 | Jazz Pharmaceuticals | 上市 | 2018-08 | |
| 长春新碱 | 急性髓细胞性白血病,黑色素瘤 | Spectrum | 上市 | 2012-08 | |
| 急性髓细胞性白血病 | 上海复旦张江 | 上市 | 2009-03 | ||
| 和厚朴酚 | 晚期非小细胞肺癌 | 成都金瑞 | 临床Ⅰ期 | 2016-05 | |
| 抗感染 | 两霉素B | 真菌感柒 | NcXstar | 上市 | 1990-08 |
| 真菌感染 | 上海上药新亚 | 上市 | 2003-08 | ||
| 真菌感染 | 北京泰德 | 临床Ⅰ期 | 2015-08 | ||
| 真菌感染 | 石药集团 | 临床Ⅲ期 | 2021-11 | ||
| 真菌感染 | 台湾东洋 | 临床Ⅰ期 | 2017-05 | ||
| 麻醉 | 硫酸吗啡 | 术后疼痛 | Pacira | 上市 | 2004-05 |
| 布尔卡因 | 术后疼痛 | Pacira | 上市 | 2011-10 | |
| 心脑血管 | 前列地尔 | 下肢动脉硬化闭塞症 | 石药集团 | 临床Ⅱ期 | 2019-09 |
| 1 | JESORKA A, ORWAR O. Liposomes: Technologies and analytical applications[J]. Annual Review of Analytical Chemistry, 2008, 1: 801-832. |
| 2 | BANGHAM A D, STANDISH M M, WATKINS J C. Diffusion of univalent ions across the lamellae of swollen phospholipids[J]. Academic Press, 1965, 13(1): 238-252. |
| 3 | ALLEN T M, CULLIS P R. Liposomal drug delivery systems: From concept to clinical applications[J]. Advanced Drug Delivery Reviews, 2013, 65(1): 36-48. |
| 4 | AKBARZADEH A, REZAEI-SADABADY R, DAVARAN S, et al. Liposome: Classification, preparation, and applications[J]. Nanoscale Research Letters, 2013, 8(1): 1-9. |
| 5 | HAMMOUD Z, GHARIB R, FOURMENTIN S, et al. New findings on the incorporation of essential oil components into liposomes composed of lipoid S100 and cholesterol[J]. International Journal of Pharmaceutics, 2019, 561: 161-170. |
| 6 | GREGORIADIS G, RYMAN B E. Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases[J]. Biochemical Journal, 1971, 124(5): 58-58. |
| 7 | 姜倩, 岳冬, 顾忠伟. 脂质体传递系统的研究进展及临床应用概况[J]. 中国材料进展, 2017, 36(11): 813-826,851. |
| JIANG Qian, YUE Dong, GU Zhongwei. Advance in research and clinical application of liposomal delivery systems[J]. Materials China, 2017, 36(11): 813-826,851. | |
| 8 | FELGNER P L, GADEK T R, HOLM M, et al. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure[J]. Proceedings of the National Academy of Sciences of the United States of America, 1987, 84(21): 7413-7417. |
| 9 | KIM B K, BAE Y U, DOH K O, et al. The synthesis of cholesterol-based cationic lipids with trimethylamine head and the effect of spacer structures on transfection efficiency[J]. Bioorganic & Medicinal Chemistry Letters, 2011, 21(12): 3734-3737. |
| 10 | NICULESCU-DUVAZ D, HEYES J, SPRINGER C J. Structure-activity relationship in cationic lipid mediated gene transfection[J]. Current Medicinal Chemistry, 2003, 10(14): 1233-1261. |
| 11 | RIDEAU E, DIMOVA R, SCHWILLE P, et al. Liposomes and polymersomes: a comparative review towards cell mimicking[J]. Chemical Society Reviews, 2018, 47(23): 8572-8610. |
| 12 | STURM L, POKLAR ULRIH N. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols[J]. International Journal of Molecular Sciences, 2021, 22(12): 6547. |
| 13 | XIA Y, XU C, ZHANG X, et al. Liposome-based probes for molecular imaging: from basic research to the bedside[J]. Nanoscale, 2019, 11(13): 5822-5838. |
| 14 | BAUMGART T, HESS S T, WEBB W W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension[J]. Nature, 2003, 425(6960): 821-824. |
| 15 | LEE J H, AGARWAL V, BOSE A, et al. Transition from unilamellar to bilamellar vesicles induced by an amphiphilic biopolymer[J]. Physical Review Letters, 2006, 96(4): 048102. |
| 16 | LASIC D D. Liposomes: From physics to applications[M]. Amsterdam: Elsevier, 1993. |
| 17 | BOLINGER P Y, STAMOU D, VOGEL H. Integrated nanoreactor systems: Triggering the release and mixing of compounds inside single vesicles[J]. Journal of the American Chemical Society, 2004, 126(28): 8594-8595. |
| 18 | KISAK E T, COLDREN B, EVANS C, et al. The vesosome—A multicompartment drug delivery vehicle[J]. Current Medicinal Chemistry, 2004, 11(2): 199-219. |
| 19 | HALLAN S S, SGUIZZATO M, ESPOSITO E, et al. Challenges in the physical characterization of lipid nanoparticles[J]. Pharmaceutics, 2021, 13(4): 549. |
| 20 | PAJEAN M, HERBAGE D. Effect of collagen on liposome permeability[J]. International Journal of Pharmaceutics, 1993, 91(2-3): 209-216. |
| 21 | KIM H J, LEE C M, LEE Y B, et al. Preparation and mucoadhesive test of CSA-loaded liposomes with different characteristics for the intestinal lymphatic delivery[J]. Biotechnology and Bioprocess Engineering, 2005, 10(6): 516-521. |
| 22 | KELLER C A, KASEMO B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance[J]. Biophysical Journal, 1998, 75(3): 1397-1402. |
| 23 | GOLDBERG R, KLEIN J. Liposomes as lubricants: Beyond drug delivery[J]. Chemistry & Physics of Lipids, 2012, 165(4): 374-381. |
| 24 | TOKSOZ S, MAMMADOV R, TEKINAY A B, et al. Electrostatic effects on nanofiber formation of self-assembling peptide amphiphiles[J]. Journal of Colloid and Interface Science, 2011, 356(1): 131-137. |
| 25 | ISRAELACHVILI J N, MITCHELL D J, NINHAM B W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers [J]. Journal of the Chemical Society Faraday Transactions, 1976, 72(24): 1525-1568. |
| 26 | 李建辉. 囊泡的形成与界面分子相互作用的二次谐波研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. |
| LI Jianhui. Study on the formation of vesicle and its interaction with molecules at the interface with second harmonic generation[D]. Harbin: Harbin Institute of Technology, 2019. | |
| 27 | 孙慧萍, 张国喜, 程光, 等. 脂质体药物的制备方法及临床应用[J]. 中国医药工业杂志, 2019, 50(10): 1160-1171. |
| SUN Huiping, ZHANG Guoxi, CHENG Guang, et al. Manufacturing and clinical application of liposomal drugs[J]. Chinese Journal of Pharmaceuticals, 2019, 50(10): 1160-1171. | |
| 28 | 侯丽芬, 谷克仁, 吴永辉. 不同制剂脂质体制备方法的研究进展[J]. 河南工业大学学报(自然科学版), 2016, 37(5): 118-124. |
| HOU Lifen, GU Keren, WU Yonghui. Research progress of the preparation methods of liposome about different formulations[J]. Journal of Henan University of Technology (Natural Science Edition), 2016, 37(5): 118-124. | |
| 29 | 汤洁, 刘仁发, 戴志飞. 多功能脂质体递药系统[J]. 化学进展, 2018, 30(11): 1669-1680. |
| TANG Jie, LIU Renfa, DAI Zhifei. Multifunctional liposomal drug delivery technology[J]. Progress in Chemistry, 2018, 30(11): 1669-1680. | |
| 30 | GOUDA A, SAKR O S, NASR M, et al. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications[J]. Journal of Drug Delivery Science and Technology, 2021, 61: 102174. |
| 31 | SUR S, FRIES A C, KINZLER K W, et al. Remote loading of pre-encapsulated drugs into stealth liposomes[J]. Proceedings of the National Academy of Sciences, 2014, 111(6): 2283-2291. |
| 32 | 杨贵兰, 李文军, 王春华. 靶向脂质体的研究进展[J]. 药学研究, 2020, 39(5): 289-293,307. |
| YANG Guilan, LI Wenjun, WANG Chunhua. Research progress of targeted liposomes[J]. Journal of Pharmaceutical Research, 2020, 39(5): 289-293, 307. | |
| 33 | KELLY C, JEFFERIES C, CRYAN S A. Targeted liposomal drug delivery to monocytes and macrophages[J]. Journal of Drug Delivery, 2011: 727241. |
| 34 | AHMAND M Z, AHMAD J, ALASMARY M Y, et al. Emerging advances in cationic liposomal cancer nanovaccines: Opportunities and challenges[J]. Immunotherapy, 2021, 13(6): 491-507. |
| 35 | TANG Q, YU B, GAO L L, et al. Stimuli responsive nanoparticles for controlled anti-cancer drug release[J]. Current Medicinal Chemistry, 2018, 25(16): 1837-1866. |
| 36 | SUBHAN M A, YALAMARTY S S K, FILIPCZAK N, et al. Recent advances in tumor targeting via EPR effect for cancer treatment[J]. Journal of Personalized Medicine, 2021, 11(6): 571. |
| 37 | VAN DER KOOG L, GANDEK T B, NAGELKERKE A. Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization[J]. Advanced Healthcare Materials, 2022, 11(5): 2100639. |
| 38 | GABIZON A, SHMEEDA H, BARENHOLZ Y. Pharmacokinetics of pegylated liposomal doxorubicin[J]. Clinical Pharmacokinetics, 2003, 42(5): 419-436. |
| 39 | SUK J S, XU Q, KIM N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery[J]. Advanced Drug Delivery Reviews, 2016, 99: 28-51. |
| 40 | PATTNI B S, CHUPIN V V, TORCHILIN V P. New developments in liposomal drug delivery[J]. Chemical Reviews, 2015, 115(19): 10938-10966. |
| 41 | KHAN A A, ALLEMAILEM K S, ALMATROODI S A, et al. Recent strategies towards the surface modification of liposomes: An innovative approach for different clinical applications[J]. 3 Biotech, 2020, 10(4): 163. |
| 42 | DE LIMA P H C, BUTERA A P, CABECA L F, et al. Liposome surface modification by phospholipid chemical reactions[J]. Chemistry and Physics of Lipids, 2021, 237: 105084. |
| 43 | YAN Z Q, WANG F, WEN Z Y, et al. LyP-1-conjugated PEGylated liposomes: A carrier system for targeted therapy of lymphatic metastatic tumor[J]. Journal of Controlled Release, 2011, 157(1): 118-125. |
| 44 | MARUYAMA K, TAKIZAWA T, YUDA T, et al. Targetability of novel immunoliposomes modified with amphipathic poly(ethylene glycol)s conjugated at their distal terminals to monoclonal antibodies[J]. Biochimica et Biophysica Acta, 1995, 1234(1): 74-80. |
| 45 | ERIKA B K, NILL B, JORGEN C, et al. Development of EGF-conjugated liposomes for targeted delivery of boronated DNA-binding agents[J]. Bioconjugate Chemistry, 2002, 13(4): 737-743. |
| 46 | ANKIT J, JAIN S K. Stimuli-responsive smart liposomes in cancer targeting[J]. Current Drug Targets, 2018, 19(3): 259-270. |
| 47 | 马秋燕, 林华庆, 张静, 等. 磁靶向热敏脂质体在抗肿瘤靶向治疗中的新进展[J]. 中国医药工业杂志, 2019, 50(12): 1405-1412. |
| MA Qiuyan, LIN Huaqing, ZHANG Jing, et al. New research progress of magnetic thermosensitive liposomes in tumor targeting therapy[J]. Chinese Journal of Pharmaceuticals, 2019, 50(12): 1405-1412. | |
| 48 | 黄丹华. 阿霉素磁靶向光敏脂质体的制备及其在肿瘤光热-化疗联合治疗中的应用[D]. 镇江: 江苏大学, 2017. |
| HUANG Danhua. Preparation of doxorubicin magnetic targeting-photosensitive liposomes and its application in combined photothermal-chemotherapy of cancer[D]. Zhenjiang: Jiangsu University, 2017. | |
| 49 | RAY S, CHENG C A, CHEN W, et al. Magneti cheating stimulated cargo release with dose control using multifunctional MR and thermosensitive liposome[J]. Nanotheranostics, 2019, 3(2): 166-178. |
| 50 | DRUMMOND D C, ZIGNANI M, LEROUX J C. Current status of pH-sensitive liposomes in drug delivery[J]. Progress in Lipid Research, 2000, 39(5): 409-460. |
| 51 | PALIWAL S R, PALIWAL R, PAL H C, et al. Estrogen-anchored pH-sensitive liposomes as nanomodule designed for site-specific delivery of doxorubicin in breast cancer therapy[J]. Molecular Pharmaceutics, 2012, 9(1): 176-186. |
| 52 | KE W D, LI J J, ZHAO K J, et al. Modular design and facile synthesis of enzyme-responsive peptide-linked block copolymers for efficient delivery of doxorubicin[J]. Biomacromolecules, 2016, 17(10): 3268-3276. |
| 53 | FRANCIS R J, SHARMA S K, SPRINGER C, et al. A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours[J]. British Journal of Cancer, 2002, 87(6): 600-607. |
| 54 | PENG Z, FANG E H, WANG C X, et al. Construction of novel thermosensitive magnetic cationic liposomes as a drug and gene co-delivery system[J]. Journal of Nanoscience and Nanotechnology, 2015, 15(5): 3823-3833. |
| 55 | NEEDHAM D, ANYARAMBHATLA G, KONG G, et al. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model[J]. Cancer Research, 2000, 60(5): 1197-1201. |
| 56 | NIE S M. Understanding and overcoming major barriers in cancer nanomedicine[J]. Nanomedicine, 2010, 5(4): 523-531. |
| 57 | HOU X C, ZAKS T, LANGER R, et al. Lipid nanoparticles for mRNA delivery[J]. Nature Reviews Materials, 2021, 6(12): 11-17. |
| 58 | MITCHELL M J, BILLINGSLEY M M, HALEY R M, et al. Engineering precision nanoparticles for drug delivery[J]. Nature Reviews Drug discovery, 2021, 20(2):101-124. |
| 59 | CORACA-HUBER D C, FILLE M, HAUSDORFER J, et al. Efficacy of antibacterial bioactive glass S53P4 against S. aureus biofilms grown on titanium discs in vitro [J]. Journal of Orthopaedic Research, 2014, 32(1): 175-182. |
| 60 | KOYNOVA R, TENCHOV B, WANG L, et al. Hydrophobic moiety of cationic lipids strongly modulates their transfection activity[J]. Molecular Pharmaceutics, 2009, 6(3): 951-959. |
| 61 | LI L, SONG H M, LUO K, et al. Gene transfer efficacies of serum-resistant amino acids-based cationic lipids: dependence on headgroup, lipoplex stability and cellular uptake[J]. International Journal of Pharmaceutics, 2011, 408(1-2): 183-190. |
| 62 | TENCHOV R, BIRD R, CURTZE A E, et al. Lipid nanoparticles—From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement[J]. ACS Nano, 2021, 15(11): 16982-17015. |
| 63 | PINNAPIREDDY S R, DUSE L L, STREHLOW B, et al. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown[J]. Colloids and Surfaces B: Biointerfaces, 2017, 158: 93-101. |
| 64 | SUI L, WANG M, HAN Q Q, et al. A novel lipidoid-microRNA formulation promotes calvarial bone regeneration[J]. Biomaterials, 2018, 177: 88-97. |
| 65 | KURIMOTO S, YOSHINAGA N, IGARASHI K, et al. PEG-OligoRNA hybridization of mRNA for developing sterically stable lipid nanoparticles toward in vivo administration[J]. Molecules, 2019, 24(7): 1303. |
| 66 | VARYPATAKI E M, VAN DER MAADEN K, BOUWSTRA J, et al. Cationic liposomes loaded with a synthetic long peptide and poly(I:C): a defined adjuvanted vaccine for induction of antigen-specific T cell cytotoxicity[J]. The AAPS Journal, 2015, 17(1): 216-226. |
| 67 | DAI C C, YANG J R, HUSSEIN W M, et al. Polyethylenimine: An intranasal adjuvant for liposomal peptide-based subunit vaccine against Group A Streptococcus [J]. ACS Infectious Diseases, 2020, 6(9): 2502-2512. |
| 68 | ZHANG Y L, HO S H, LI B Z, et al. Modulating the tumor microenvironment with new therapeutic nanoparticles: A promising paradigm for tumor treatment[J]. Medicinal Research Reviews, 2020, 40(3): 1084-1102. |
| 69 | RUTTALA H B, KO Y T. Liposomal co-delivery of curcumin and albumin/paclitaxel nanoparticle for enhanced synergistic antitumor efficacy[J]. Colloids and Surfaces B: Biointerfaces, 2015, 128: 419-426. |
| 70 | FANCIULLINO R, MOLLARD S, GIACOMETTI S, et al. In vitro and in vivo evaluation of lipofufol, a new triple stealth liposomal formulation of modulated 5-FU: Impact on efficacy and toxicity[J]. Pharmaceutical Research, 2013, 30(5): 1281-1290. |
| 71 | DICKO A, KWAK S, FRAZIER A A, et al. Biophysical characterization of a liposomal formulation of cytarabine and daunorubicin[J]. International Journal of Pharmaceutics, 2010, 391(1/2): 248-259. |
| 72 | MAROOF H, ISLAM F, DONG L Fet al. Liposomal delivery of miR-34b-5p induced cancer cell death in thyroid carcinoma[J]. Cells, 2018, 7(12): 265-281. |
| [1] | 刘源岗1,2,郑琪瑶1,王士斌1,2. 盐酸米托蒽醌多囊脂质体的制备工艺优化及性能[J]. 化工进展, 2013, 32(06): 1395-1400. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||