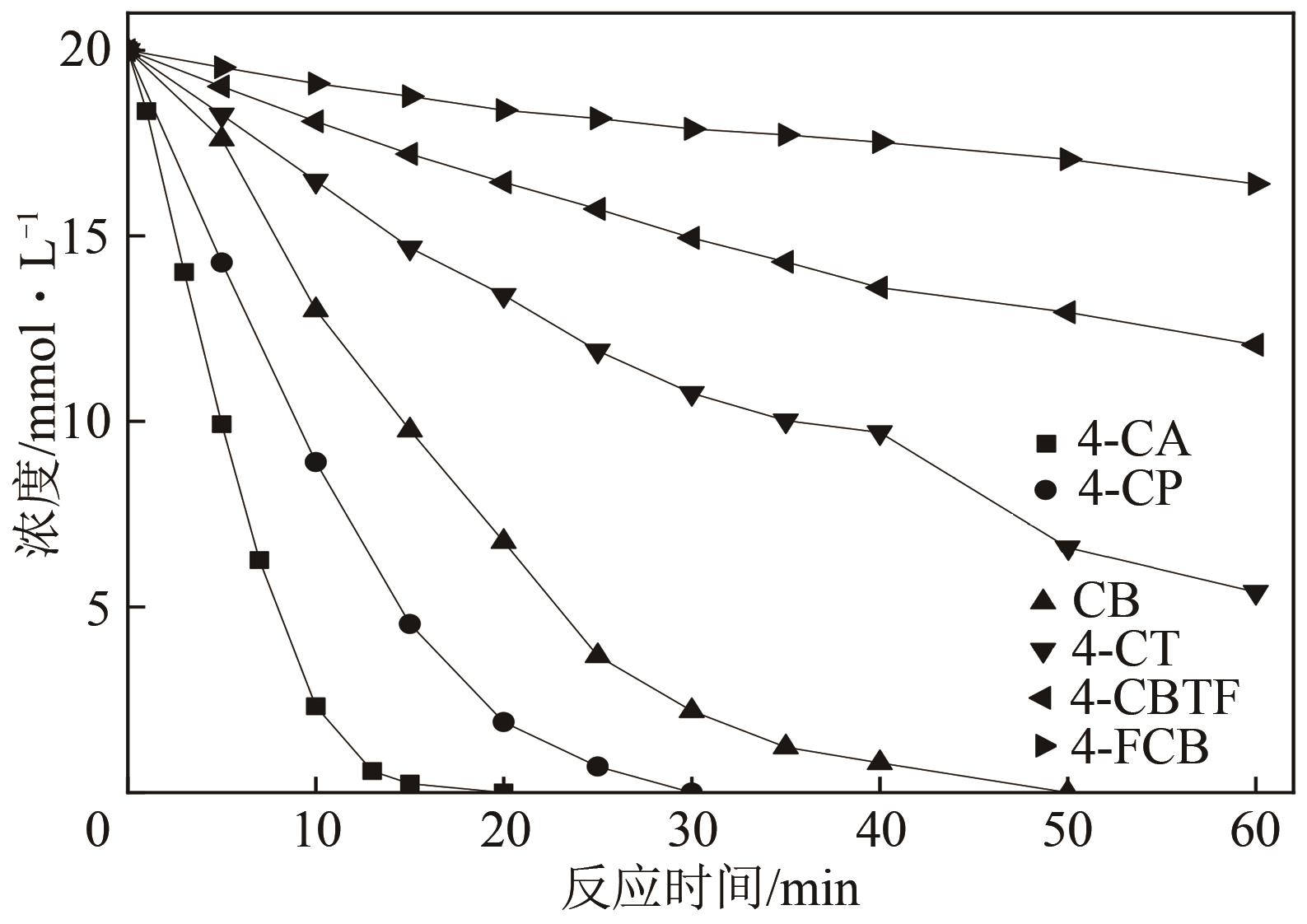
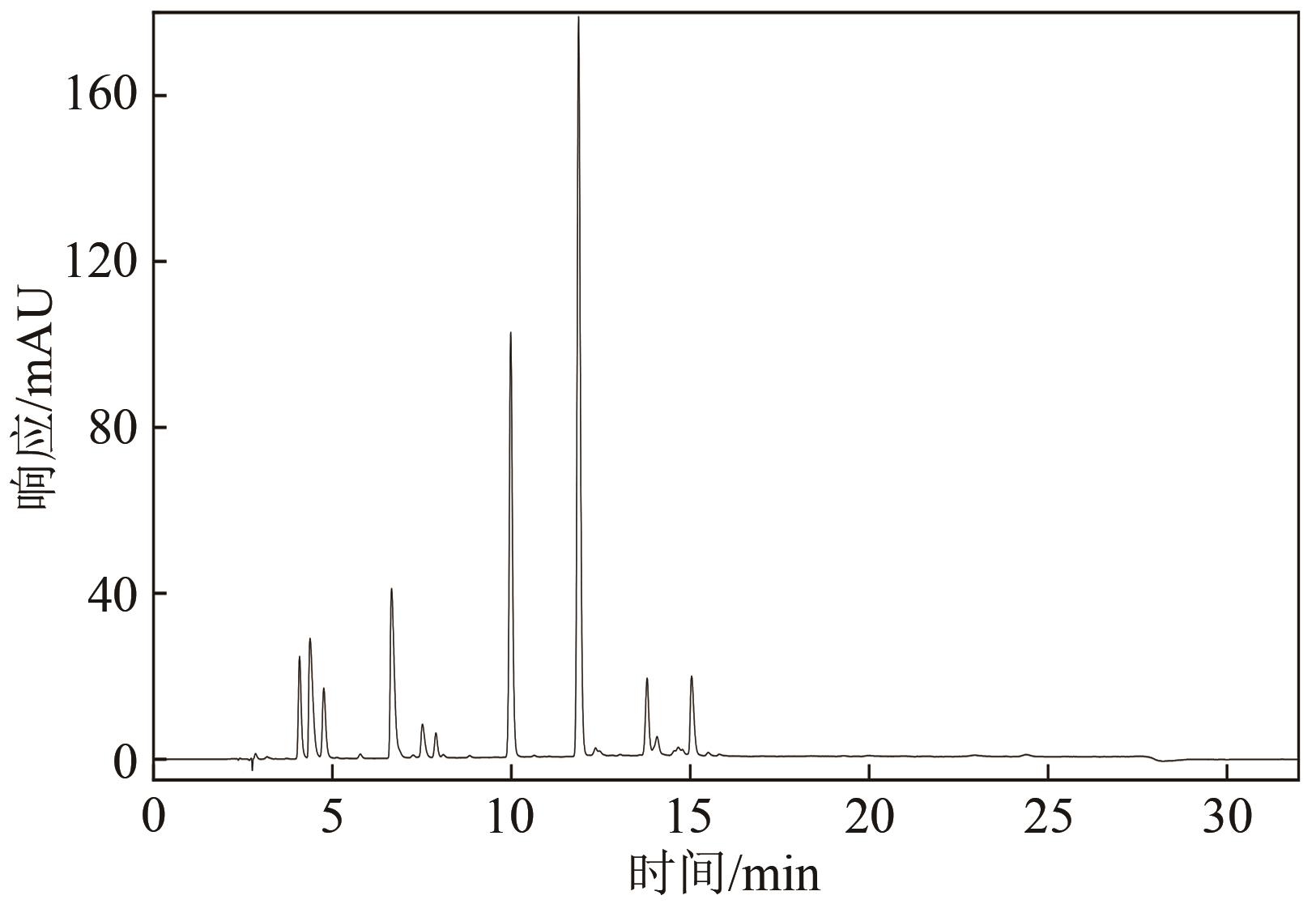
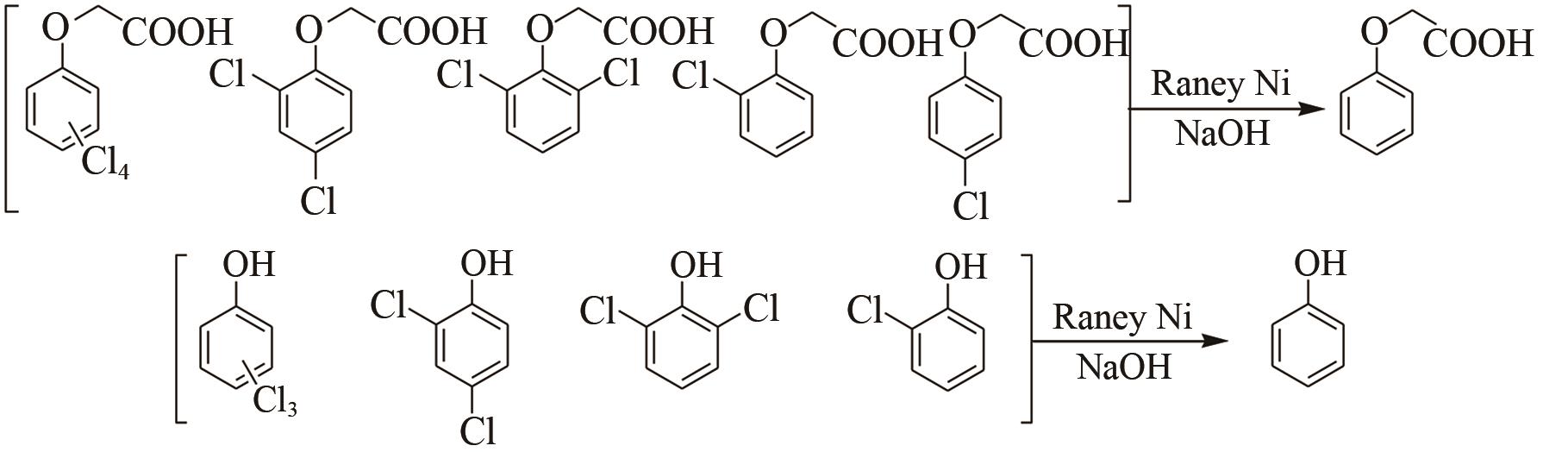
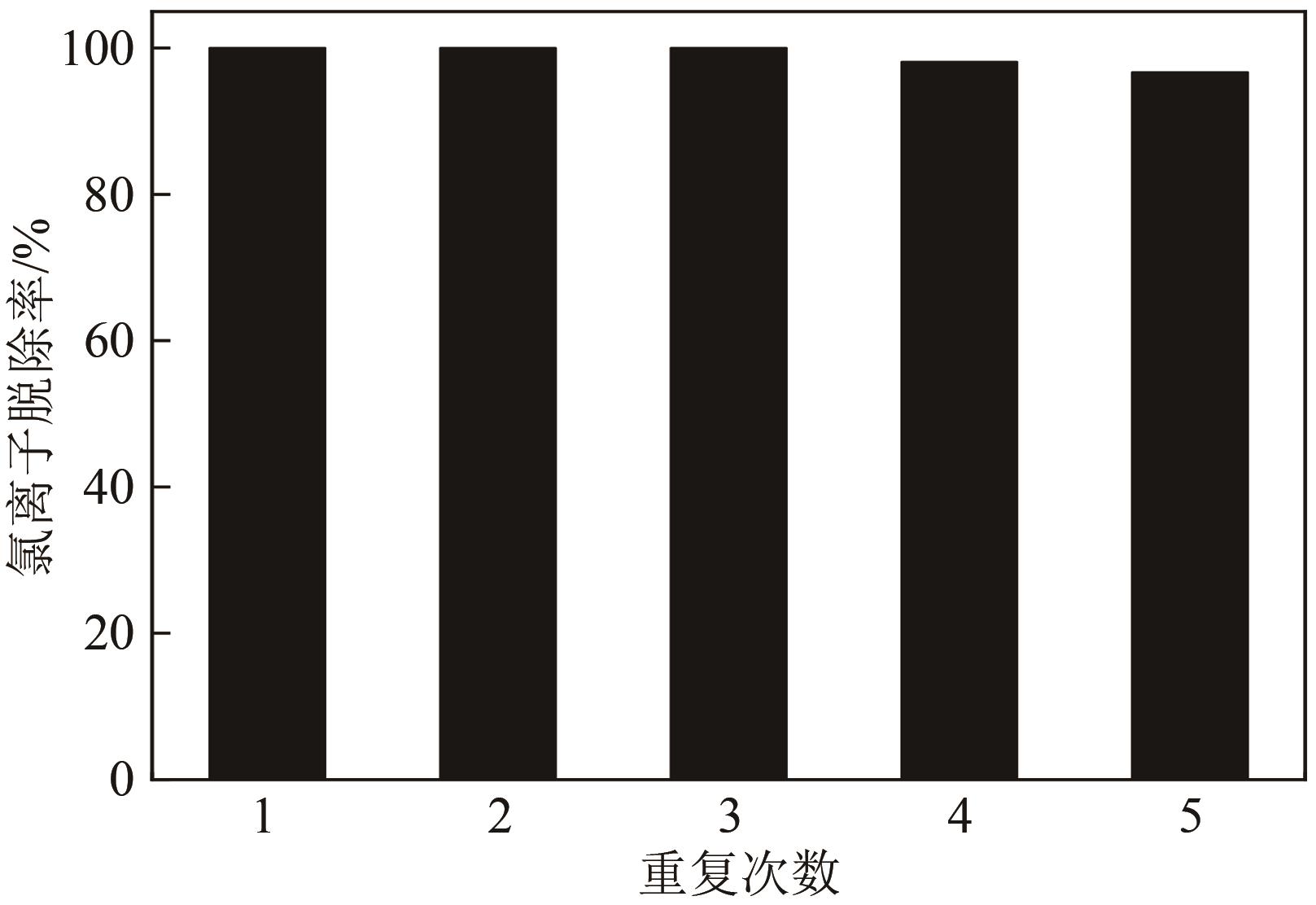
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (7): 4003-4010.DOI: 10.16085/j.issn.1000-6613.2020-1520
• Resources and environmental engineering • Previous Articles Next Articles
Effective reductive dechlorination of chlorophenols over Raney Ni catalyst under mild conditions
JIA Yuejuan1( ), LI Xiaohan2, MA Xuanxuan2, XIE Qingming3, LIU Ying2, LIU Sujing2, XIA Chuanhai1,2(
), LI Xiaohan2, MA Xuanxuan2, XIE Qingming3, LIU Ying2, LIU Sujing2, XIA Chuanhai1,2( )
)
- 1.School of Chemistry and Materials Science, Ludong University, Yantai 264025, Shandong, China
2.School of Resources and Environmental Engineering, Ludong University, Yantai 264025, Shandong, China
3.Weifang Intellectual Property Protection Center, Weifang 261101, Shandong, China
-
Received:2020-08-03Revised:2020-11-13Online:2021-07-19Published:2021-07-06 -
Contact:XIA Chuanhai
Raney Ni高效催化氯酚类污染物还原脱氯降解
贾月娟1( ), 李晓涵2, 马宣宣2, 谢清明3, 刘莺2, 刘苏静2, 夏传海1,2(
), 李晓涵2, 马宣宣2, 谢清明3, 刘莺2, 刘苏静2, 夏传海1,2( )
)
- 1.鲁东大学化学与材料科学学院,山东 烟台 264025
2.鲁东大学资源与环境工程学院,山东 烟台 264025
3.潍坊市知识产权保护中心,山东 潍坊 261101
-
通讯作者:夏传海 -
作者简介:贾月娟(1995—),女,硕士研究生,研究方向为污染物控制。E-mail:jiayj1225@163.com 。 -
基金资助:国家自然科学基金(22006063);山东省自然科学基金青年项目(ZR2020QB145);固体废弃物资源化利用福建省高校工程研究中心开放基金(LYGF-201803);中科院海岸带环境过程与生态修复重点实验室开放课题(2018KFJJ07)
CLC Number:
Cite this article
JIA Yuejuan, LI Xiaohan, MA Xuanxuan, XIE Qingming, LIU Ying, LIU Sujing, XIA Chuanhai. Effective reductive dechlorination of chlorophenols over Raney Ni catalyst under mild conditions[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 4003-4010.
贾月娟, 李晓涵, 马宣宣, 谢清明, 刘莺, 刘苏静, 夏传海. Raney Ni高效催化氯酚类污染物还原脱氯降解[J]. 化工进展, 2021, 40(7): 4003-4010.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1520
| 催化剂性质 | 表征值 |
|---|---|
| Ni质量分数/% | 90 |
| Al质量分数/% | ≤7 |
| Mo质量分数/% | 3 |
| pH | 8~11 |
| 平均粒径/μm | 50 |
| 活性(以H2产量计)/mL·min-1·g-1 | ≥5 |
| 催化剂性质 | 表征值 |
|---|---|
| Ni质量分数/% | 90 |
| Al质量分数/% | ≤7 |
| Mo质量分数/% | 3 |
| pH | 8~11 |
| 平均粒径/μm | 50 |
| 活性(以H2产量计)/mL·min-1·g-1 | ≥5 |
| t/min | 流动相A/% | 流动相B/% | 流动相C/% |
|---|---|---|---|
| 0 | 55 | 5 | 40 |
| 7 | 35 | 15 | 50 |
| 12 | 20 | 15 | 65 |
| 25 | 20 | 15 | 65 |
| 26 | 55 | 5 | 40 |
| 32 | 55 | 5 | 40 |
| t/min | 流动相A/% | 流动相B/% | 流动相C/% |
|---|---|---|---|
| 0 | 55 | 5 | 40 |
| 7 | 35 | 15 | 50 |
| 12 | 20 | 15 | 65 |
| 25 | 20 | 15 | 65 |
| 26 | 55 | 5 | 40 |
| 32 | 55 | 5 | 40 |
| 序号 | 保留时间/min | 化合物 | 主要离子(m/z) | 质量浓度/g·kg-1 | 质量分数/% |
|---|---|---|---|---|---|
| 1 | 3.23 | PA | 151,137,93 | 1.23 | 0.32 |
| 2 | 4.12 | 2-CPA | 185,127,92,79 | 26.37 | 5.64 |
| 3 | 4.38 | 2,6-DCPA | 219,161,127,91 | 46.74 | 8.43 |
| 4 | 4.77 | 4-CPA | 185,127,92,79 | 21.98 | 4.70 |
| 5 | 5.56 | 苯酚 | 93,79 | N.D. | 0 |
| 6 | 6.69 | 2,4-DCPA | 219,161,127,91 | 64.77 | 11.68 |
| 7 | 7.52 | TriCP | 195,161,125,91 | 11.79 | 2.38 |
| 8 | 7.89 | 2-CP | 127,91,65 | 3.53 | 1.10 |
| 9 | 8.78 | 4-CP | 127,91,65 | N.D. | 0 |
| 10 | 9.98 | 2,6-DCP | 161,125,91 | 73.60 | 17.99 |
| 11 | 11.88 | 2,4-DCP | 161,125,91 | 154.37 | 37.75 |
| 12 | 13.83 | 2,4,6-TCP | 195,161,123,91 | 24.15 | 4.87 |
| 13 | 15.04 | TetraCPA | 287,253,195,161 | 38.66 | 5.14 |
| 序号 | 保留时间/min | 化合物 | 主要离子(m/z) | 质量浓度/g·kg-1 | 质量分数/% |
|---|---|---|---|---|---|
| 1 | 3.23 | PA | 151,137,93 | 1.23 | 0.32 |
| 2 | 4.12 | 2-CPA | 185,127,92,79 | 26.37 | 5.64 |
| 3 | 4.38 | 2,6-DCPA | 219,161,127,91 | 46.74 | 8.43 |
| 4 | 4.77 | 4-CPA | 185,127,92,79 | 21.98 | 4.70 |
| 5 | 5.56 | 苯酚 | 93,79 | N.D. | 0 |
| 6 | 6.69 | 2,4-DCPA | 219,161,127,91 | 64.77 | 11.68 |
| 7 | 7.52 | TriCP | 195,161,125,91 | 11.79 | 2.38 |
| 8 | 7.89 | 2-CP | 127,91,65 | 3.53 | 1.10 |
| 9 | 8.78 | 4-CP | 127,91,65 | N.D. | 0 |
| 10 | 9.98 | 2,6-DCP | 161,125,91 | 73.60 | 17.99 |
| 11 | 11.88 | 2,4-DCP | 161,125,91 | 154.37 | 37.75 |
| 12 | 13.83 | 2,4,6-TCP | 195,161,123,91 | 24.15 | 4.87 |
| 13 | 15.04 | TetraCPA | 287,253,195,161 | 38.66 | 5.14 |
| 12 | 陈阳雯, 刘蕊, 姚洁, 等. La掺杂钛基氧化物电极降解水中2,4-二氯酚的研究[J]. 环境科学与技术, 2018, 41(1): 94-98. |
| CHEN Yangwen, LIU Rui, YAO Jie, et al. Degradation of 2,4-dichlorophenol by Ti-based oxide electrodes doped with La[J]. Environmental Science and Technology, 2018, 41(1): 94-98. | |
| 13 | YANG Z, ZHANG X, PU S, et al. Novel Fenton-like system (Mg/Fe-O2) for degradation of 4-chlorophenol[J]. Environmental Pollution, 2019, 250: 906-913. |
| 14 | HADI S, TAHERI E, AMIN M M, et al. Synergistic degradation of 4-chlorophenol by persulfate and oxalic acid mixture with heterogeneous Fenton like system for wastewater treatment: adaptive neuro-fuzzy inference systems modeling[J]. Journal of Environmental Management, 2020, 268: 110678. |
| 15 | 张云飞, 杨波, 张鸿, 等. 负载型催化剂用于卤代有机污染物氢解去除[J]. 化学进展, 2013, 25(12): 2159-2168. |
| ZHANG Yunfei, YANG Bo, ZHANG Hong, et al. Degradation of halogenated organic contaminants with hydrodehalogenation using supported catalysts[J]. Progress in Chemistry, 2013, 25(12): 2159-2168. | |
| 16 | XIONG J, MA Y, YANG W, et al. Rapid, highly efficient and stable catalytic hydrodechlorination of chlorophenols over novel Pd/CNTs-Ni foam composite catalyst in continuous-flow[J]. Journal of Hazardous Materials, 2018, 355: 89-95. |
| 17 | XU F, DENG S, XU J, et al. Highly active and stable Ni-Fe bimetal prepared by ball milling for catalytic hydrodechlorination of 4-chlorophenol[J]. Environmental Science and Technology, 2012, 46: 4576-4582. |
| 18 | MA X, LIU Y, LI X, et al. Water: the most effective solvent for liquid-phase hydrodechlorination of chlorophenols over Raney Ni catalyst[J]. Applied Catalysis B: Environmental, 2015, 165: 351-359. |
| 19 | RAUT S S, SHETTY R, RAJU N M, et al. Screening of zero valent mono/bimetallic catalysts and recommendation of Raney Ni (without reducing agent) for dechlorination of 4-chlorophenol[J]. Chemosphere, 2020, 250: 126298. |
| 20 | WANG W, NIU J, YANG Z. An efficient reduction of unsaturated bonds and halogen-containing groups by nascent hydrogen over Raney Ni catalyst[J]. Journal of Hazardous Materials, 2020, 389: 121912. |
| 21 | URBANO F J, MARINAS J M. Hydrogenolysis of organohalogen compounds over palladium supported catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2001, 173(1/2): 329-345. |
| 22 | CONCIBIDO N C, OKUDA T, NISHIJIMA W, et al. Deactivation and reactivation of Pd/C catalyst used in repeated batch hydrodechlorination of PCE[J]. Applied Catalysis B: Environmental, 2007, 71(1/2): 64-69. |
| 23 | XIA C, LIU Y, ZHOU S, et al. The Pd-catalyzed hydrodechlorination of chlorophenols in aqueous solutions under mild conditions: a promising approach to practical use in wastewater[J]. Journal of Hazardous Materials, 2009, 169(1/2/3): 1029-1033. |
| 24 | MA X, LIU Y, LIU S, et al. Water-promoted catalytic hydrodechlorination of transformer oil-contained PCBs in liquid system under mild conditions[J]. Applied Catalysis B: Environmental, 2014, 144: 580-587. |
| 1 | CHENG R, ZHOU W, WANG J, et al. Dechlorination of pentachlorophenol using nanoscale Fe/Ni particles: role of nano-Ni and its size effect[J]. Journal of Hazardous Materials, 2010, 180(1/2/3): 79-85. |
| 2 | GARBA Z N, ZHOU W, LAWAN I, et al. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: a review[J]. Journal of Environmental Management, 2019, 241: 59-75. |
| 3 | 兰丽娟, 刘莺, 杜芳林. Pd/CNTs对4-氯苯酚的液相催化加氢去氯[J].化工进展, 2017, 36(6): 2171-2176. |
| 25 | 马宣宣. Pd/C和Raney Ni催化有机卤代物的液相高效加氢脱卤研究[D]. 烟台: 中国科学院烟台海岸带研究所, 2015. |
| Ma Xuanxuan. Highly efficient liquid-phase hydrodehalogenation of halogenated aromatic compounds over Pd/C and Raney Ni catalysts[D]. Yantai: Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, 2015. | |
| 26 | MA X, ZHOU S, YANG C, et al. The influence of triethylamine on the hydrodechlorination reactivity of chlorophenols over Raney Ni catalyst[J]. Catalysis Communications, 2010, 12(4): 282-285. |
| 27 | YONEDA T, TAKIDO T, KONUMA K. Hydrodechlorination reactivity of para-substituted chlorobenzenes over platinum/carbon catalyst[J]. Journal of Molecular Catalysis A: Chemical, 2007, 265(1-2): 80-89. |
| 28 | AREMENDÍA M A, BORÁU V, GARCÍA I M, et al. Liquid-phase hydrodehalogenation of substituted chlorobenzenes over palladium supported catalysts[J]. Applied Catalysis B: Environmental, 2003, 43(1): 71-79. |
| 29 | KONUMA K, KAMEDA N. Effect of substituents on the hydrodechlorination reactivity of para-substituted chlorobenzenes[J]. Journal of Molecular Catalysis A: Chemical, 2002, 178(1-2): 239-251. |
| 30 | WU W H, XU J, OHNISHI R. Complete hydrodechlorination of chlorobenzene and its derivatives over supported nickel catalysts under liquid phase conditions[J]. Applied Catalysis B: Environmental, 2005, 60(1/2): 129-137. |
| 31 | GRYGLEWICZ S, PIECHOCKI W. Hydrodechlorination of dichlorobenzenes and their derivatives over Ni-Mo/C catalyst: kinetic analysis and effect of molecular structure of reactant[J]. Chemosphere, 2011, 83(3): 334-339. |
| 3 | LAN Lijuan, LIU Ying, DU Fanglin. Liquid-phase catalytic hydrodechlorination of 4-chlorophenol over Pd/CNTs[J]. Chemical Industry and Engineering Progress, 2017, 36(6): 2171-2176. |
| 4 | WEI D, ZHAO C, KHAN A, et al. Sorption mechanism and dynamic behavior of graphene oxide as an effective adsorbent for the removal of chlorophenol based environmental-hormones: a DFT and MD simulation study[J]. Chemical Engineering Journal, 2019, 375: 121964. |
| 5 | YOUNIS S A, MOTAWEA E A, MOUSTAFA Y M, et al. A strategy for the efficient removal of chlorophenols in petrochemical wastewater by organophilic and aminated silica@alginate microbeads: taguchi optimization and isotherm modeling based on partition coefficient[J]. Journal of Hazardous Materials, 2020, 397: 122792. |
| 6 | ADEYEMI I, SULAIMAN R, ALMAZROUI M, et al. Removal of chlorophenols from aqueous media with hydrophobic deep eutectic solvents: experimental study and COSMO RS evaluation[J]. Journal of Molecular Liquids, 2020, 311: 113180. |
| 7 | 李燕妮, 陈泉源, 周娟, 等. 石墨烯杂氮载Pd催化剂对2,4-二氯酚的液相催化加氢脱氯[J]. 中国环境科学, 2017, 37(2): 577-583. |
| LI Yanni, CHEN Quanyuan, ZHOU Juan, et al. The liquid phase catalytic hydrogenation of 2,4-dichlorophenol over Pd catalyst supported on nitrogen-doped graphene[J]. China Environmental Science, 2017, 37(2): 577-583. | |
| 8 | 刘楚琛, 阎秀兰, 刘琼枝, 等. Fenton试剂和活化过硫酸钠氧化降解土壤中的二氯酚和三氯酚[J]. 环境工程学报, 2018, 12(6): 1749-1758. |
| LIU Chuchen, YAN Xiulan, LIU Qiongzhi, et al. Oxidative degradation of dichlorophenol and trichlorophenol in soils by Fenton reagent and activated persulfate[J]. Chinese Journal of Environmental Engineering, 2018, 12(6): 1749-1758. | |
| 9 | 阮霞, 刘红, 马驰, 等. 铜质量比对Fe/Cu还原2,4-二氯酚影响及还原途径[J]. 环境科学与技术, 2017, 40(5): 43-48. |
| RUAN Xia, LIU Hong, MA Chi, et al. Effects of mass ratio of copper on reduction of 2,4-dichlorophenol by Fe/Cu and reduction pathway[J]. Environmental Science and Technology, 2017, 40(5): 43-48. | |
| 10 | MA X, LIU S, LIU Y, et al. Promoted liquid-phase hydrodechlorination of chlorophenol over Raney Ni via controlling base: performance, mechanism, and application[J]. Chemosphere, 2020, 242: 125202. |
| 11 | BAO T, DAMTIE M M, HOSSEINZADEH A, et al. Catalytic degradation of P-chlorophenol by muscovite-supported nano zero valent iron composite: synthesis, characterization, and mechanism studies[J]. Applied Clay Science, 2020, 195: 105735. |
| [1] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [2] | WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410. |
| [3] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [4] | SHAO Boshi, TAN Hongbo. Simulation on the enhancement of cryogenic removal of volatile organic compounds by sawtooth plate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 84-93. |
| [5] | GENG Yuanze, ZHOU Junhu, ZHANG Tianyou, ZHU Xiaoyu, YANG Weijuan. Homogeneous/heterogeneous coupled combustion of heptane in a partially packed bed burner [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4514-4521. |
| [6] | GAO Yanjing. Analysis of international research trend of single-atom catalysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4667-4676. |
| [7] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [8] | LI Dongze, ZHANG Xiang, TIAN Jian, HU Pan, YAO Jie, ZHU Lin, BU Changsheng, WANG Xinye. Research progress of NO x reduction by carbonaceous substances for denitration in cement kiln [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4882-4893. |
| [9] | WANG Chen, BAI Haoliang, KANG Xue. Performance study of high power UV-LED heat dissipation and nano-TiO2 photocatalytic acid red 26 coupling system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4905-4916. |
| [10] | HUANG Yufei, LI Ziyi, HUANG Yangqiang, JIN Bo, LUO Xiao, LIANG Zhiwu. Research progress on catalysts for photocatalytic CO2 and CH4 reforming [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4247-4263. |
| [11] | WU Haibo, WANG Xilun, FANG Yanxiong, JI Hongbing. Progress of the development and application of 3D printing catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3956-3964. |
| [12] | XU Wei, LI Kaijun, SONG Linye, ZHANG Xinghui, YAO Shunhua. Research progress of photocatalysis and co-electrochemical degradation of VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3520-3531. |
| [13] | GONG Pengcheng, YAN Qun, CHEN Jinfu, WEN Junyu, SU Xiaojie. Properties and mechanism of eriochrome black T degradation by carbon nanotube-cobalt ferrite composites activated persulfate [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3572-3581. |
| [14] | GUO Lixing, PANG Weiying, MA Keyao, YANG Jiahan, SUN Zehui, ZHANG Pan, FU Dong, ZHAO Kun. Hierarchically multilayered TiO2 with spatial pore-structure for efficient photocatalytic CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3643-3651. |
| [15] | CHU Tiantian, LIU Runzhu, DU Gaohua, MA Jiahao, ZHANG Xiao’a, WANG Chengzhong, ZHANG Junying. Preparation and chemical degradability of organoguanidine-catalyzed dehydrogenation type RTV silicone rubbers [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3664-3673. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||