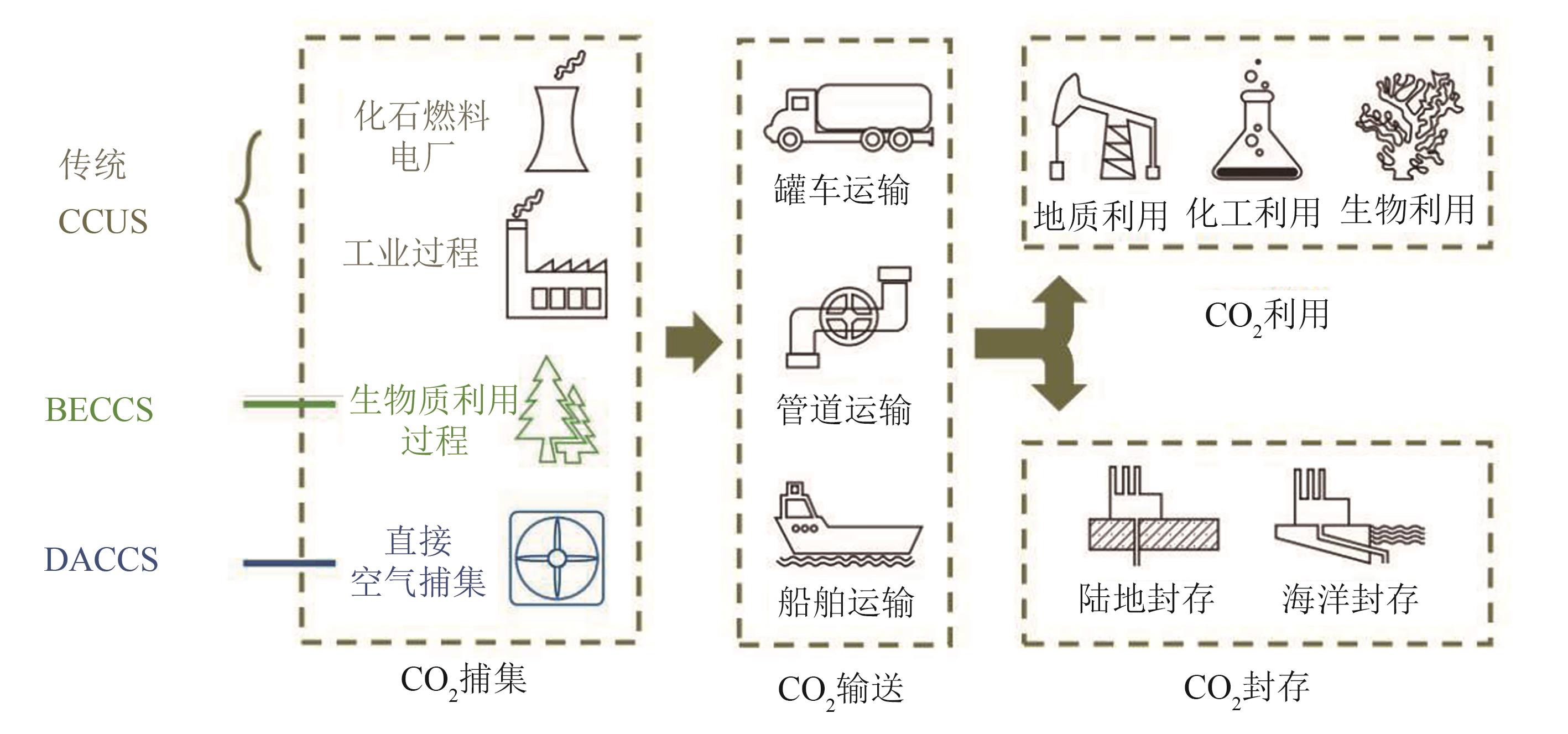
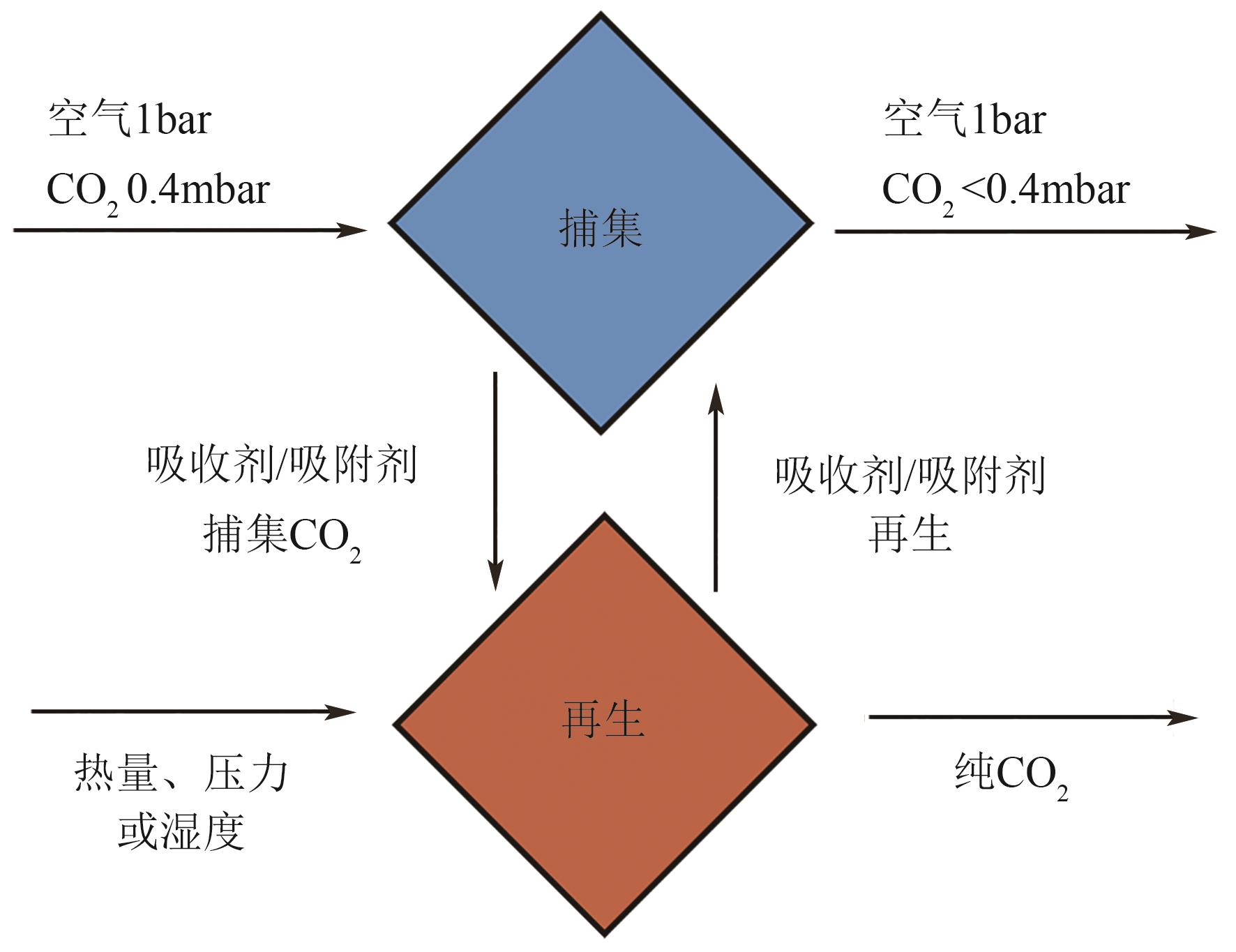
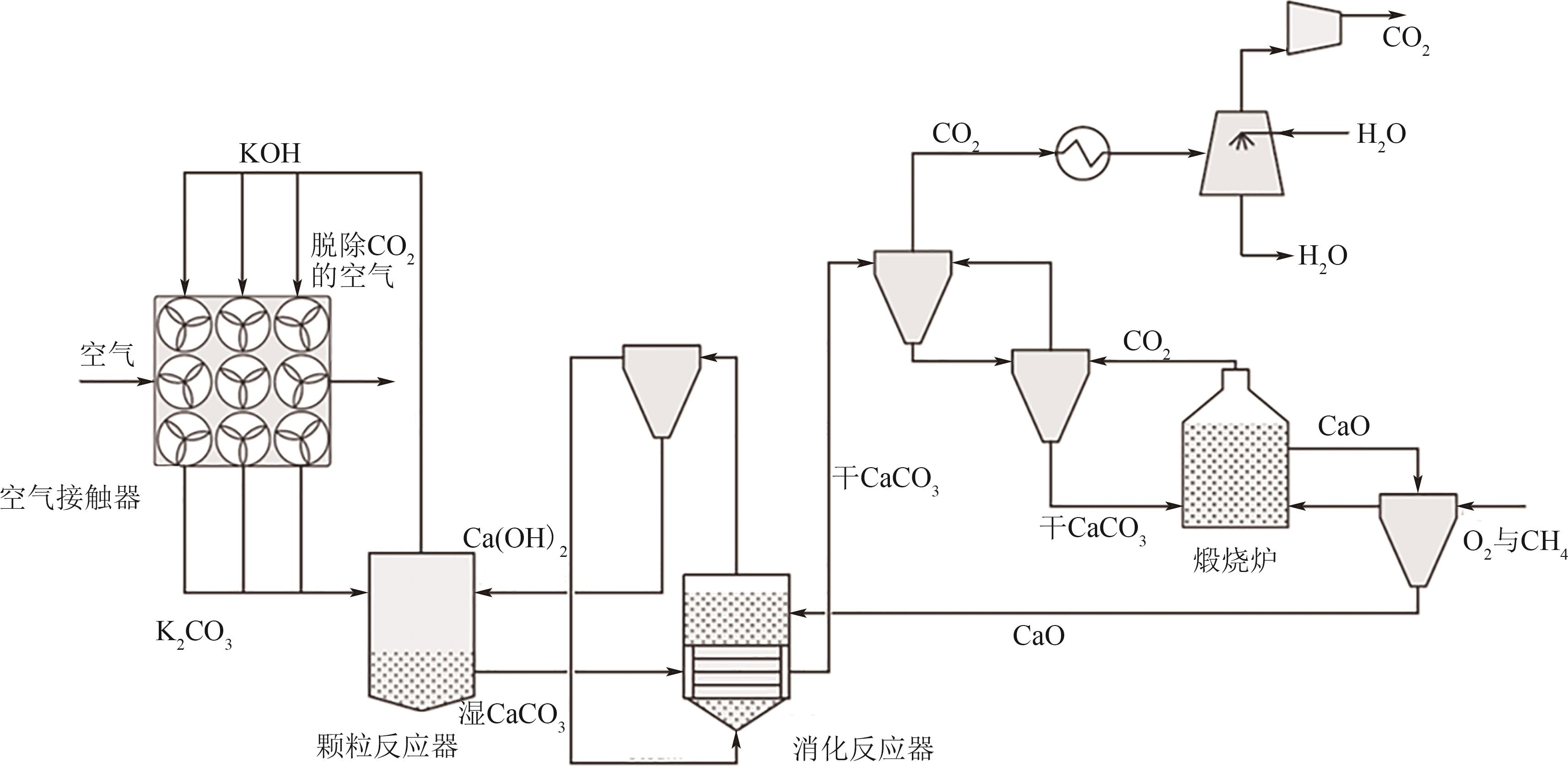
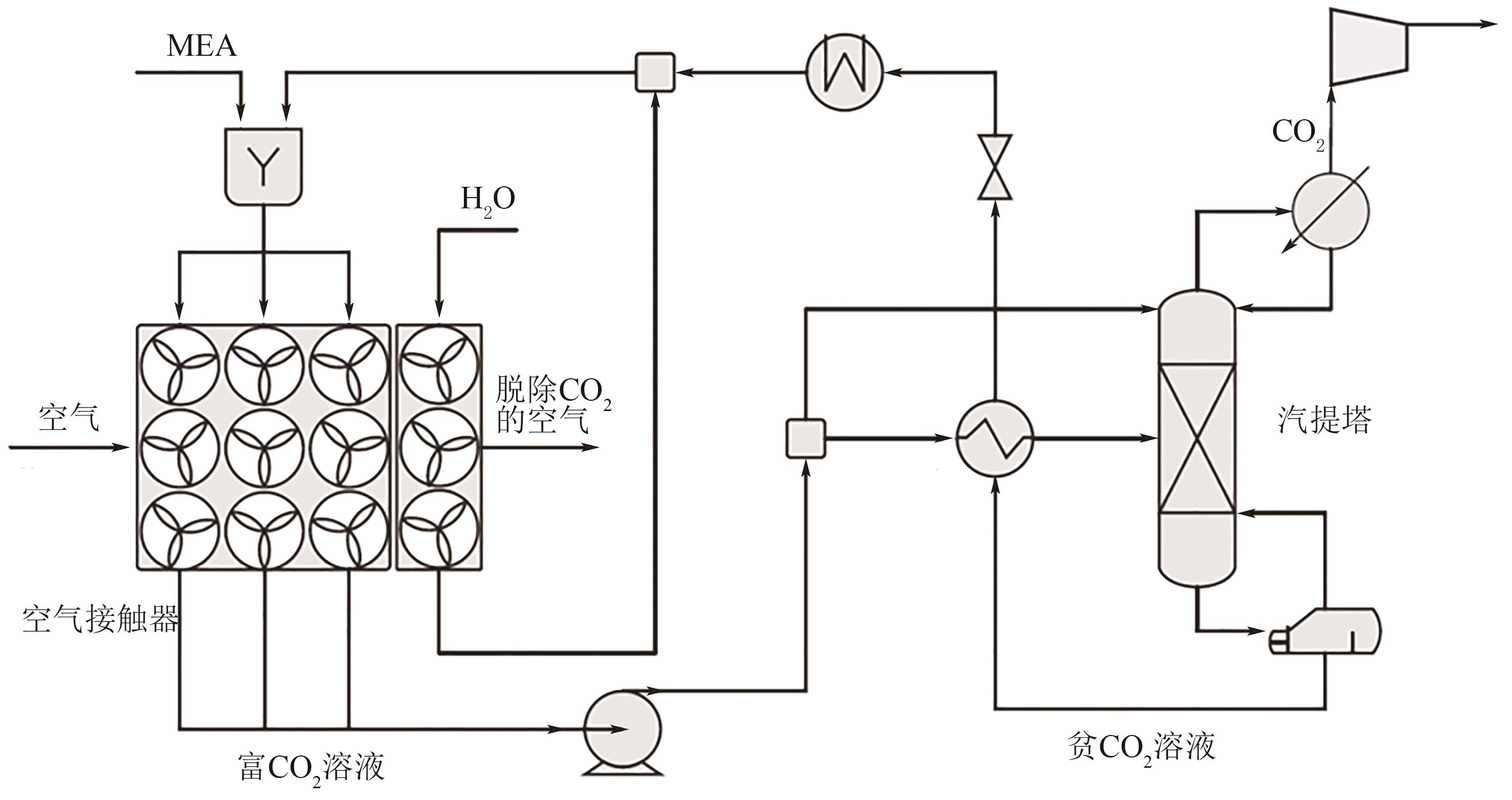
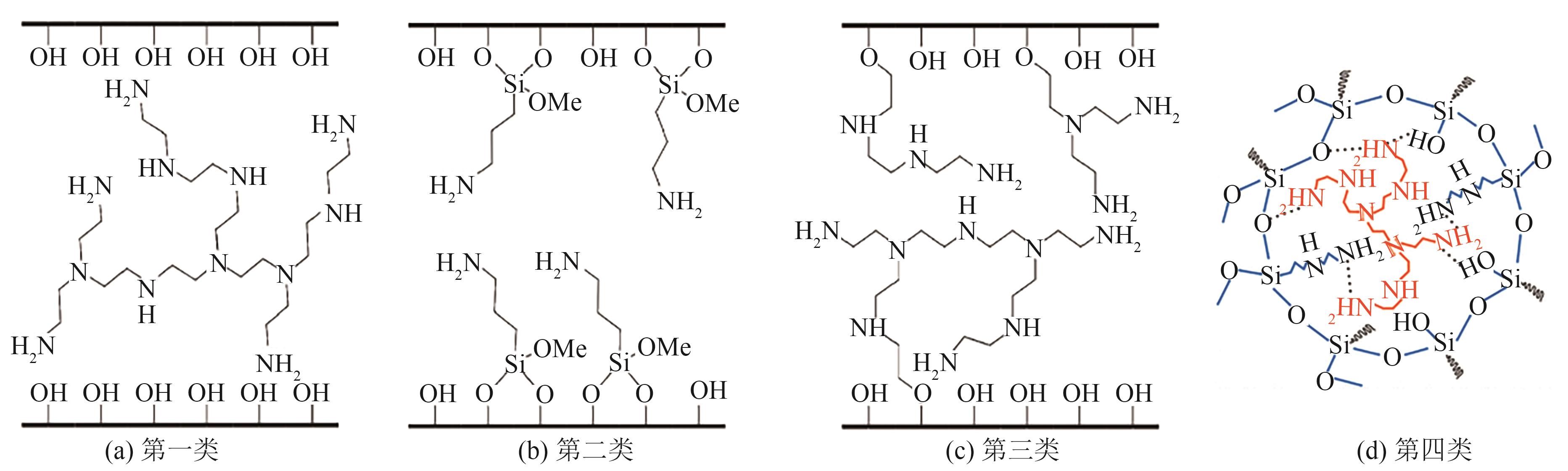
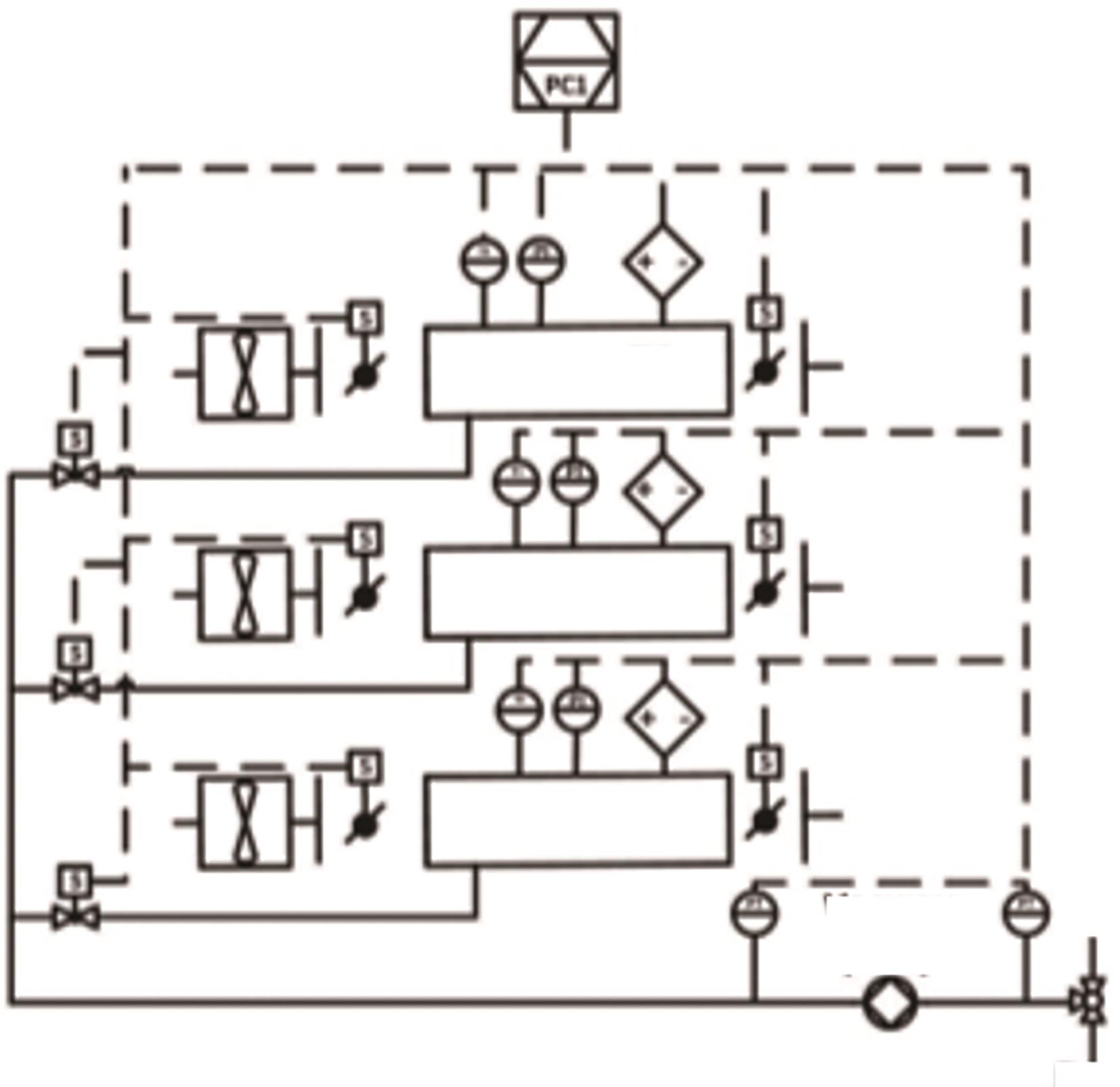
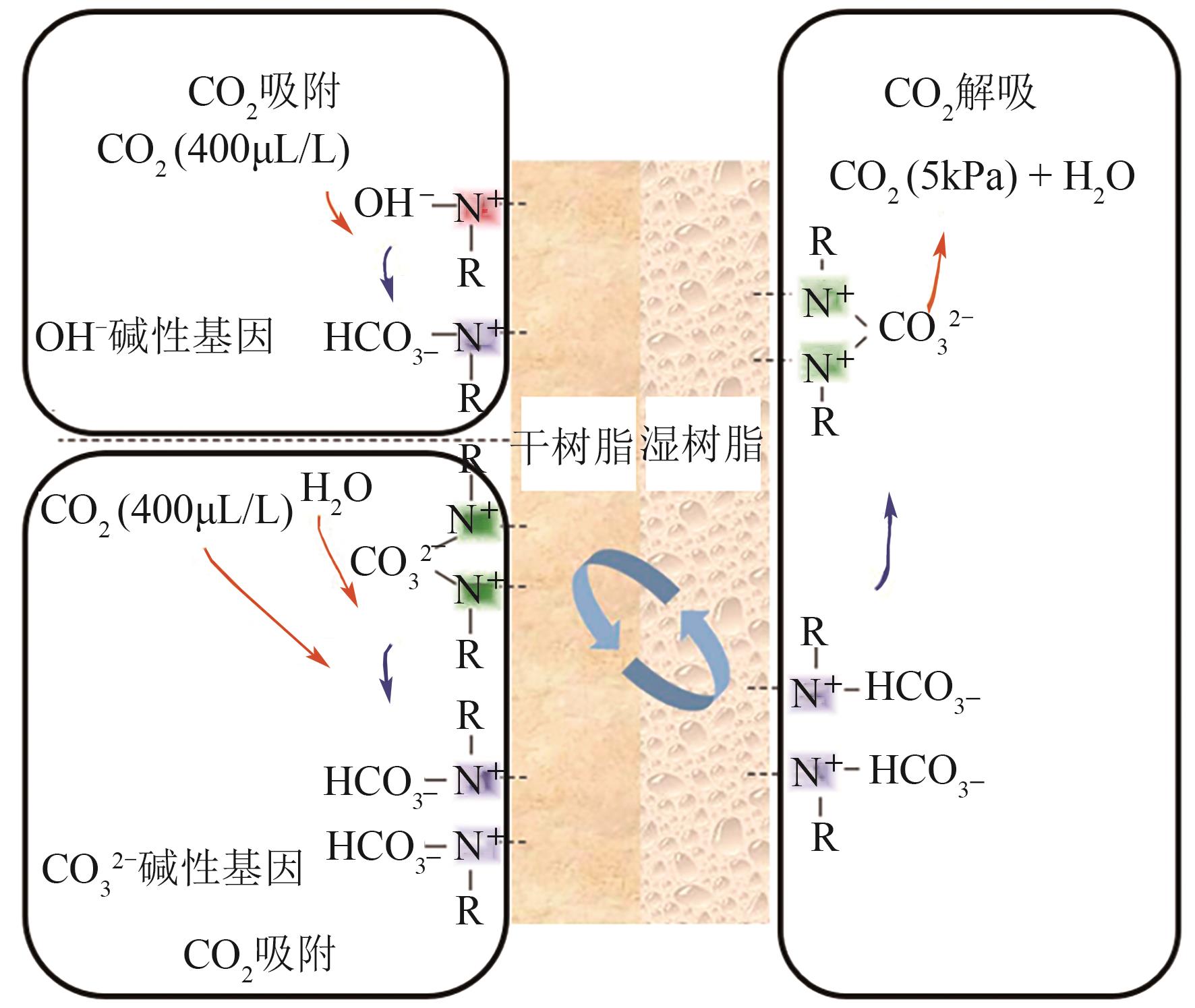
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (4): 2031-2048.DOI: 10.16085/j.issn.1000-6613.2023-0606
• Resources and environmental engineering • Previous Articles
Progress on direct air capture of carbon dioxide
LIAO Changjian1( ), ZHANG Kewei2, WANG Jing1, ZENG Xiangyu1, JIN Ping1, LIU Zhiyu1
), ZHANG Kewei2, WANG Jing1, ZENG Xiangyu1, JIN Ping1, LIU Zhiyu1
- 1.Dalian Research Institute of Petroleum and Petrochemicals, Dalian 116045, Liaoning, China
2.China Petroleum and Chemical Corporation, Beijing 100728, China
-
Received:2023-04-14Revised:2023-06-16Online:2024-05-13Published:2024-04-15 -
Contact:LIAO Changjian
直接空气捕集二氧化碳技术研究进展
廖昌建1( ), 张可伟2, 王晶1, 曾翔宇1, 金平1, 刘志禹1
), 张可伟2, 王晶1, 曾翔宇1, 金平1, 刘志禹1
- 1.中石化(大连)石油化工研究院有限公司,辽宁 大连 116045
2.中国石油化工股份有限公司,北京 100728
-
通讯作者:廖昌建 -
作者简介:廖昌建(1984—),男,硕士,研究员,研究方向为碳捕集技术开发。E-mail:liaochangjian.fshy@sinopec.com。 -
基金资助:中国石化科技开发资助项目(321095)
CLC Number:
Cite this article
LIAO Changjian, ZHANG Kewei, WANG Jing, ZENG Xiangyu, JIN Ping, LIU Zhiyu. Progress on direct air capture of carbon dioxide[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2031-2048.
廖昌建, 张可伟, 王晶, 曾翔宇, 金平, 刘志禹. 直接空气捕集二氧化碳技术研究进展[J]. 化工进展, 2024, 43(4): 2031-2048.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0606
| 所属公司 | 地点 | CO2储存或利用 | 起始时间 /年 | CO2捕集量/t∙a-1 |
|---|---|---|---|---|
| Global Thermostat | 美国 | 未知 | 2010 | 500 |
| Global Thermostat | 美国 | 未知 | 2013 | 1000 |
| Climeworks | 德国 | 利用 | 2015 | 1 |
| Carbon Engineering | 加拿大 | 利用 | 2015 | 365 |
| Climeworks | 瑞士 | 利用 | 2016 | 50 |
| Climeworks | 瑞士 | 利用 | 2017 | 900 |
| Climeworks | 冰岛 | 储存 | 2017 | 50 |
| Climeworks | 瑞士 | 利用 | 2018 | 600 |
| Climeworks | 瑞士 | 利用 | 2018 | 3 |
| Climeworks | 意大利 | 利用 | 2018 | 150 |
| Climeworks | 德国 | 利用 | 2019 | 3 |
| Climeworks | 荷兰 | 利用 | 2019 | 3 |
| Climeworks | 德国 | 利用 | 2019 | 3 |
| Climeworks | 德国 | 利用 | 2019 | 50 |
| Climeworks | 德国 | 利用 | 2020 | 50 |
| Climeworks | 德国 | 利用 | 2020 | 3 |
| Climeworks | 德国 | 利用 | 2020 | 3 |
| Climeworks | 冰岛 | 储存 | 2021 | 4000 |
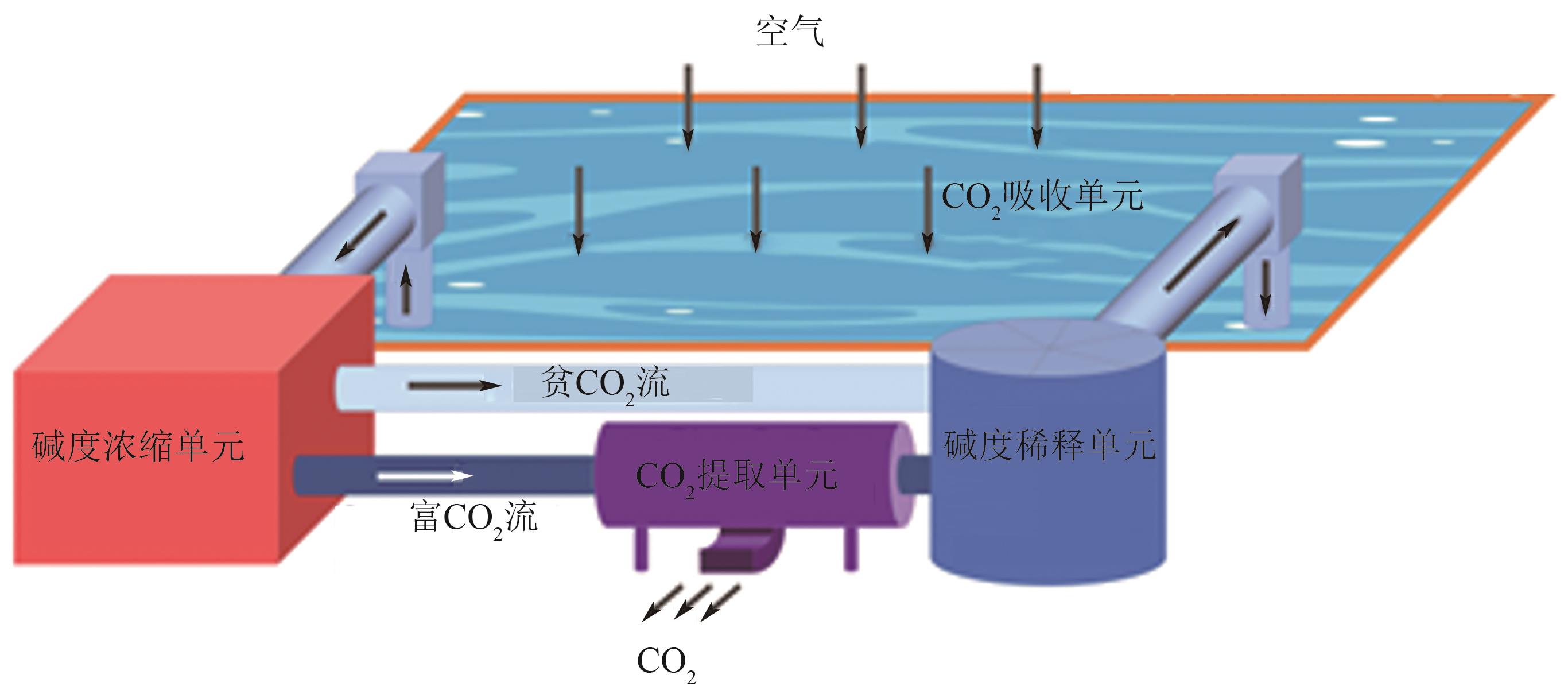
| 所属公司 | 地点 | CO2储存或利用 | 起始时间 /年 | CO2捕集量/t∙a-1 |
|---|---|---|---|---|
| Global Thermostat | 美国 | 未知 | 2010 | 500 |
| Global Thermostat | 美国 | 未知 | 2013 | 1000 |
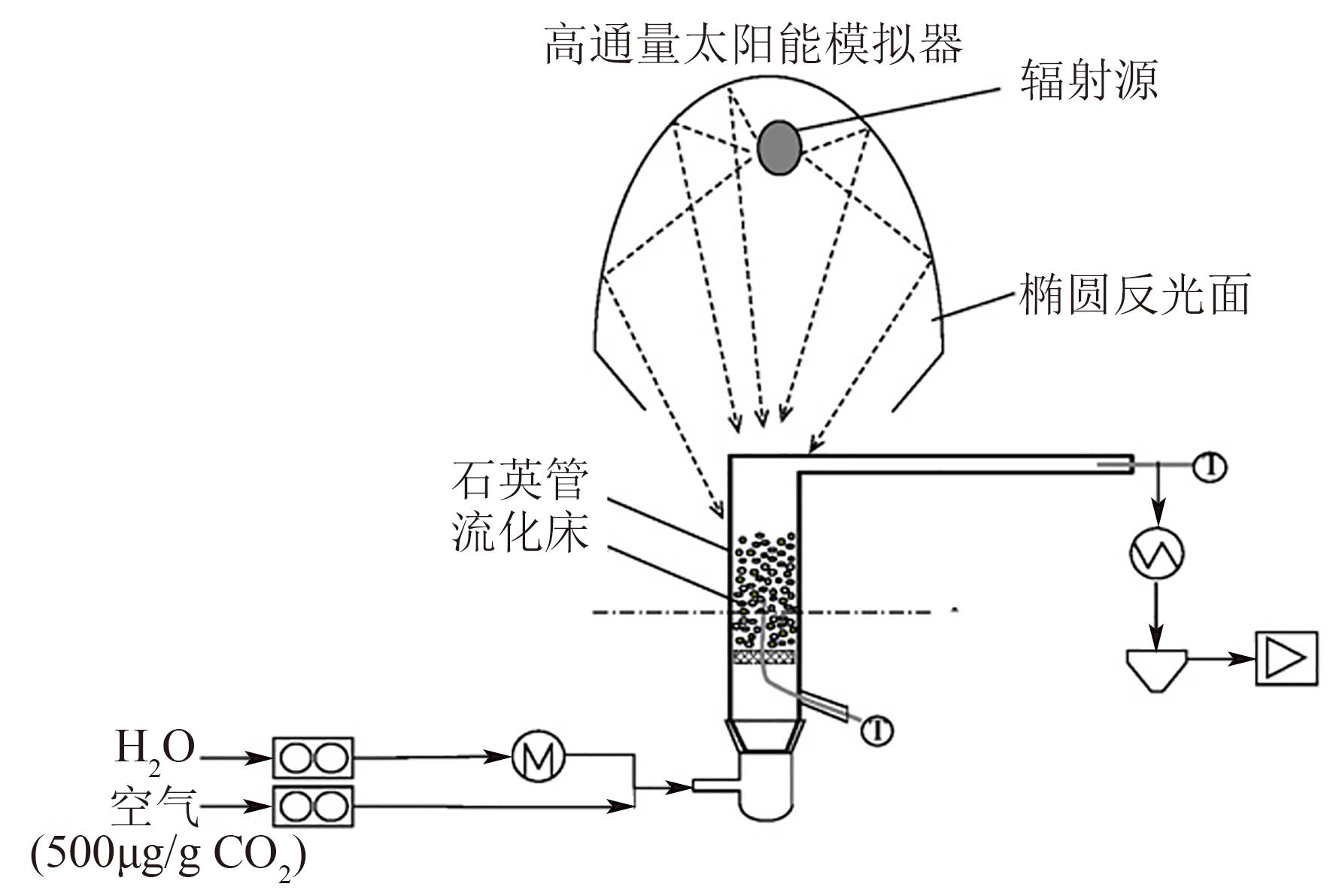
| Climeworks | 德国 | 利用 | 2015 | 1 |
| Carbon Engineering | 加拿大 | 利用 | 2015 | 365 |
| Climeworks | 瑞士 | 利用 | 2016 | 50 |
| Climeworks | 瑞士 | 利用 | 2017 | 900 |
| Climeworks | 冰岛 | 储存 | 2017 | 50 |
| Climeworks | 瑞士 | 利用 | 2018 | 600 |
| Climeworks | 瑞士 | 利用 | 2018 | 3 |
| Climeworks | 意大利 | 利用 | 2018 | 150 |
| Climeworks | 德国 | 利用 | 2019 | 3 |
| Climeworks | 荷兰 | 利用 | 2019 | 3 |
| Climeworks | 德国 | 利用 | 2019 | 3 |
| Climeworks | 德国 | 利用 | 2019 | 50 |
| Climeworks | 德国 | 利用 | 2020 | 50 |
| Climeworks | 德国 | 利用 | 2020 | 3 |
| Climeworks | 德国 | 利用 | 2020 | 3 |
| Climeworks | 冰岛 | 储存 | 2021 | 4000 |
| 载体 | 胺基 | 实验环境 | 最大吸附容量 /mmol∙g-1 | 吸附-脱附 循环条件 | 循环吸附量 /mmol∙g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| SBA-15 | TEPA(质量分数50%) | 400μg/g CO2 | 2.30(600min) | 吸附25℃, 60min; 脱附110℃, 15min | 1.72~1.66(10次循环) | [ |
| 大孔硅 | PA(10.98mmol /g) | 400μg/g CO2 | 2.65 | 10%CO2:吸附50℃,40min; 脱附110℃,10min | 3.86~3.78(120次循环) | [ |
| 介孔炭 | PEI(质量分数55%) | 400μg/g CO2 | 2.25(720min) | 吸附25℃;脱附110℃,45min | 2.25~2.18(10次循环) | [ |
| 多壁碳纳米管(NWCN) | PEI(质量分数20%乙醇) | 350μg/g CO2 | 2.12(720min) | 吸附30℃,10min;脱附90℃,20min | 1.07~0.96(10次循环) | [ |
| 纳米纤维(NFC) | AEAPDMS (4.9mmol/g) | 506μg/g CO2, RH40% | 1.39(720min) | 吸附25℃,120min; 脱附90℃,60min | 平均0.695(20次循环) | [ |
| Mg0.55Al-CO3LDHs | TEPA(质量分数67%) | 400μg/g CO2 | 3.0(180min) | 吸附25℃,100min; 脱附100℃,10min | 1.31~1.19(80次循环) | [ |
| Mg0.55Al-CO3LDHs | TEPA(质量分数67%) | 400μg/g CO2 | 3.0(180min) | 吸附25℃, 180min; 脱附100℃,15min | 2.55~2.31(20次循环) | [ |
| Mg0.55Al-CO3LDHs | TRI(6.399mmol∙g-1) | 400μg/g CO2 | 1.05(120min) | 吸附25℃, 60min; 脱附120℃,15min | 0.912(50次循环) | [ |
| 层状SiO2 | TEPA(质量分数70%) | 400μg/g CO2 | 5.2(700min) | 吸附30℃,25min; 脱附110℃,30min | 4.5~3.5(10次循环) | [ |
| Mg2(dobdc) | N2H4(6.01mmol∙g-1) | 400μg/g CO2 | 3.89 | 15%CO2:吸附40℃,40min; 脱附130℃ | 3.86(5次循环) | [ |
聚丙烯腈(PAN) 中空纤维 | TEPA(质量分数30.1%) | 470μg/g CO2, RH25% | 2.15(440min) | 吸附25℃;脱附100℃ | 2.03(20次循环) | [ |
| 载体 | 胺基 | 实验环境 | 最大吸附容量 /mmol∙g-1 | 吸附-脱附 循环条件 | 循环吸附量 /mmol∙g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| SBA-15 | TEPA(质量分数50%) | 400μg/g CO2 | 2.30(600min) | 吸附25℃, 60min; 脱附110℃, 15min | 1.72~1.66(10次循环) | [ |
| 大孔硅 | PA(10.98mmol /g) | 400μg/g CO2 | 2.65 | 10%CO2:吸附50℃,40min; 脱附110℃,10min | 3.86~3.78(120次循环) | [ |
| 介孔炭 | PEI(质量分数55%) | 400μg/g CO2 | 2.25(720min) | 吸附25℃;脱附110℃,45min | 2.25~2.18(10次循环) | [ |
| 多壁碳纳米管(NWCN) | PEI(质量分数20%乙醇) | 350μg/g CO2 | 2.12(720min) | 吸附30℃,10min;脱附90℃,20min | 1.07~0.96(10次循环) | [ |
| 纳米纤维(NFC) | AEAPDMS (4.9mmol/g) | 506μg/g CO2, RH40% | 1.39(720min) | 吸附25℃,120min; 脱附90℃,60min | 平均0.695(20次循环) | [ |
| Mg0.55Al-CO3LDHs | TEPA(质量分数67%) | 400μg/g CO2 | 3.0(180min) | 吸附25℃,100min; 脱附100℃,10min | 1.31~1.19(80次循环) | [ |
| Mg0.55Al-CO3LDHs | TEPA(质量分数67%) | 400μg/g CO2 | 3.0(180min) | 吸附25℃, 180min; 脱附100℃,15min | 2.55~2.31(20次循环) | [ |
| Mg0.55Al-CO3LDHs | TRI(6.399mmol∙g-1) | 400μg/g CO2 | 1.05(120min) | 吸附25℃, 60min; 脱附120℃,15min | 0.912(50次循环) | [ |
| 层状SiO2 | TEPA(质量分数70%) | 400μg/g CO2 | 5.2(700min) | 吸附30℃,25min; 脱附110℃,30min | 4.5~3.5(10次循环) | [ |
| Mg2(dobdc) | N2H4(6.01mmol∙g-1) | 400μg/g CO2 | 3.89 | 15%CO2:吸附40℃,40min; 脱附130℃ | 3.86(5次循环) | [ |
聚丙烯腈(PAN) 中空纤维 | TEPA(质量分数30.1%) | 470μg/g CO2, RH25% | 2.15(440min) | 吸附25℃;脱附100℃ | 2.03(20次循环) | [ |
| 技术名称 | 优点 | 缺点 |
|---|---|---|
| 碱性氢氧化物溶液DAC技术 | 技术成熟,吸收速率高 | 再生温度高、再生能耗高,损失大量水分 |
| 胺溶液DAC技术 | 吸收速率较高 | 伴随胺液挥发且再生效率较低 |
| 氨基酸盐溶液/BIGs DAC技术 | 吸收速率高,再生温度较低,溶剂损失少 | 能耗较高,捕集效果因BIGs 而异 |
| 碱度浓度变化DAC技术 | 吸收速率较高,再生温度与能耗较低,可与海水淡化研究合作 | 捕集效果与溶液浓缩技术相关联,用水量大 |
| 固体碱(土)金属DAC技术 | 吸附效率较高,再生稳定性较好 | 再生能耗较高,成本较高 |
| 固态胺吸附剂DAC技术 | 技术成熟,吸附速率快,再生温度低 | 吸附剂的热稳定性有待进一步提高 |
| MOFs材料DAC技术 | 在较低温度下有应用优势 | 捕集效果受环境中水含量影响较大,原材料成本较高 |
| 变湿吸附DAC技术 | 吸附与解吸速率高,再生温度及再生能耗较低 | 用水量大,对水质要求高,得到的CO2分压较低 |
| DACM技术 | 可同时实现CO2捕集与转化,受湿度影响较小 | 再生温度较高,催化剂起决定性作用 |
| 光诱导摆动吸附DAC技术 | 再生能耗较低 | 需在材料内嵌入光反应因子 |
| MI-DAC技术 | 再生能耗较低,可应用于海水 | 捕集效果主要取决于微生物 |
| 技术名称 | 优点 | 缺点 |
|---|---|---|
| 碱性氢氧化物溶液DAC技术 | 技术成熟,吸收速率高 | 再生温度高、再生能耗高,损失大量水分 |
| 胺溶液DAC技术 | 吸收速率较高 | 伴随胺液挥发且再生效率较低 |
| 氨基酸盐溶液/BIGs DAC技术 | 吸收速率高,再生温度较低,溶剂损失少 | 能耗较高,捕集效果因BIGs 而异 |
| 碱度浓度变化DAC技术 | 吸收速率较高,再生温度与能耗较低,可与海水淡化研究合作 | 捕集效果与溶液浓缩技术相关联,用水量大 |
| 固体碱(土)金属DAC技术 | 吸附效率较高,再生稳定性较好 | 再生能耗较高,成本较高 |
| 固态胺吸附剂DAC技术 | 技术成熟,吸附速率快,再生温度低 | 吸附剂的热稳定性有待进一步提高 |
| MOFs材料DAC技术 | 在较低温度下有应用优势 | 捕集效果受环境中水含量影响较大,原材料成本较高 |
| 变湿吸附DAC技术 | 吸附与解吸速率高,再生温度及再生能耗较低 | 用水量大,对水质要求高,得到的CO2分压较低 |
| DACM技术 | 可同时实现CO2捕集与转化,受湿度影响较小 | 再生温度较高,催化剂起决定性作用 |
| 光诱导摆动吸附DAC技术 | 再生能耗较低 | 需在材料内嵌入光反应因子 |
| MI-DAC技术 | 再生能耗较低,可应用于海水 | 捕集效果主要取决于微生物 |
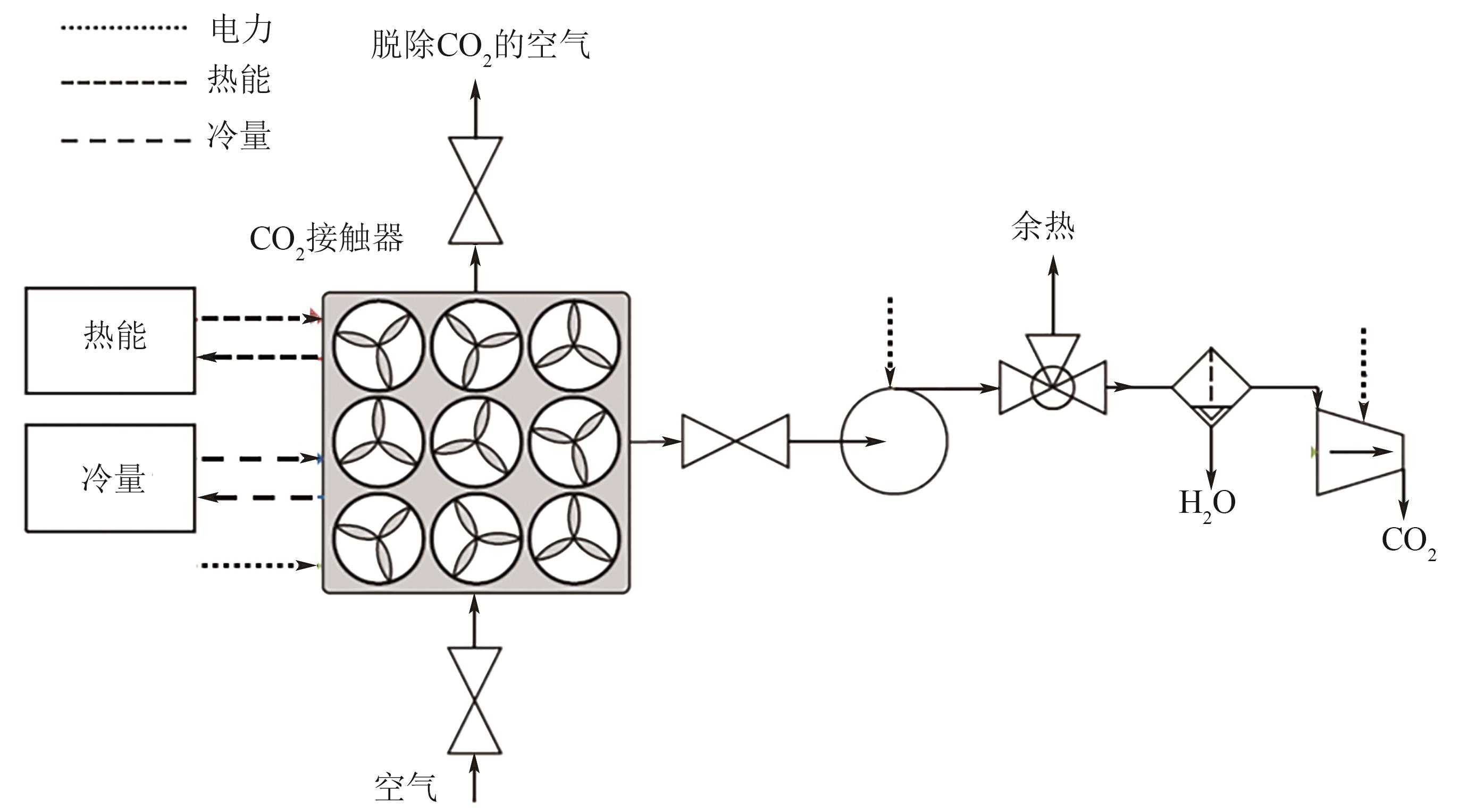
| 捕集能力 | 吸附剂/吸收剂 | 解吸温度/℃ | 能源 | 能耗 | CO2压力/bar | CO2纯度 | 成本/USD·t-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1Mt/a | KOH | 900 | 天然气 | 8.81GJ/t | 150 | 97.1% | 94~232 | [ |
| 1Mt/a | KOH | 900 | 天然气+电力 | 5.25GJ/t+366kWh/t | 150 | 97.1% | 94~232 | [ |
| 1Mt/a | KOH | 900 | 电力 | 1535kWh/t | 1 | >97% | 186 | [ |
| 0.291t/h | MEA | 123.1 | 电力 | 1452kWh/t | 2 | — | 676 | [ |
| 0.36Mt/a | K2CO3 | 80~100 | 余热 | 7.5GJ/t+694kWh/t | — | >99% | 135~177 | [ |
| 3600t/a | K2CO3 | 80~100 | 余热 | 7.5GJ/t+694kWh/t | — | >99% | 203~244 | [ |
| — | CaO | 875 | 太阳能 | 240.9 GJ/t | — | — | — | [ |
| — | K2CO3/γ-Al2O3 | 150 | 热能+机械能 | 7.3GJ/t+0.27GJ/t | — | — | — | [ |
| 300t/a | 胺基 | 100 | 余热 | 5.4~7.2GJ/t+200~300Wh/t | — | 99.9% | 预计大规模75 | [ |
| — | 氨基聚合物 | 85~95 | 蒸汽 | 4.2~5.1GJ/t+150~260Wh/t | — | >98.5% | <113 | [ |
| — | 氨基聚合物 | 75 | 低温蒸汽 | — | — | >98.5% | ≤50 | [ |
| 140g/d | MOFs | 80,真空 | 余热 | — | — | 70%~80% | 34~350 | [ |
| 166.08t/h | 变湿吸附剂+胺液 | — | 太阳能 | 306GJ/t | — | 97% | 93.1 | [ |
| 365t/a | 变湿吸附剂 | 变湿 | 电力 | 316kWh/t | — | — | 99 (预期<30) | [ |
| — | 变湿吸附剂 | 45 | 余热 | 0.81GJ/t | — | 3% | 34.68 | [ |
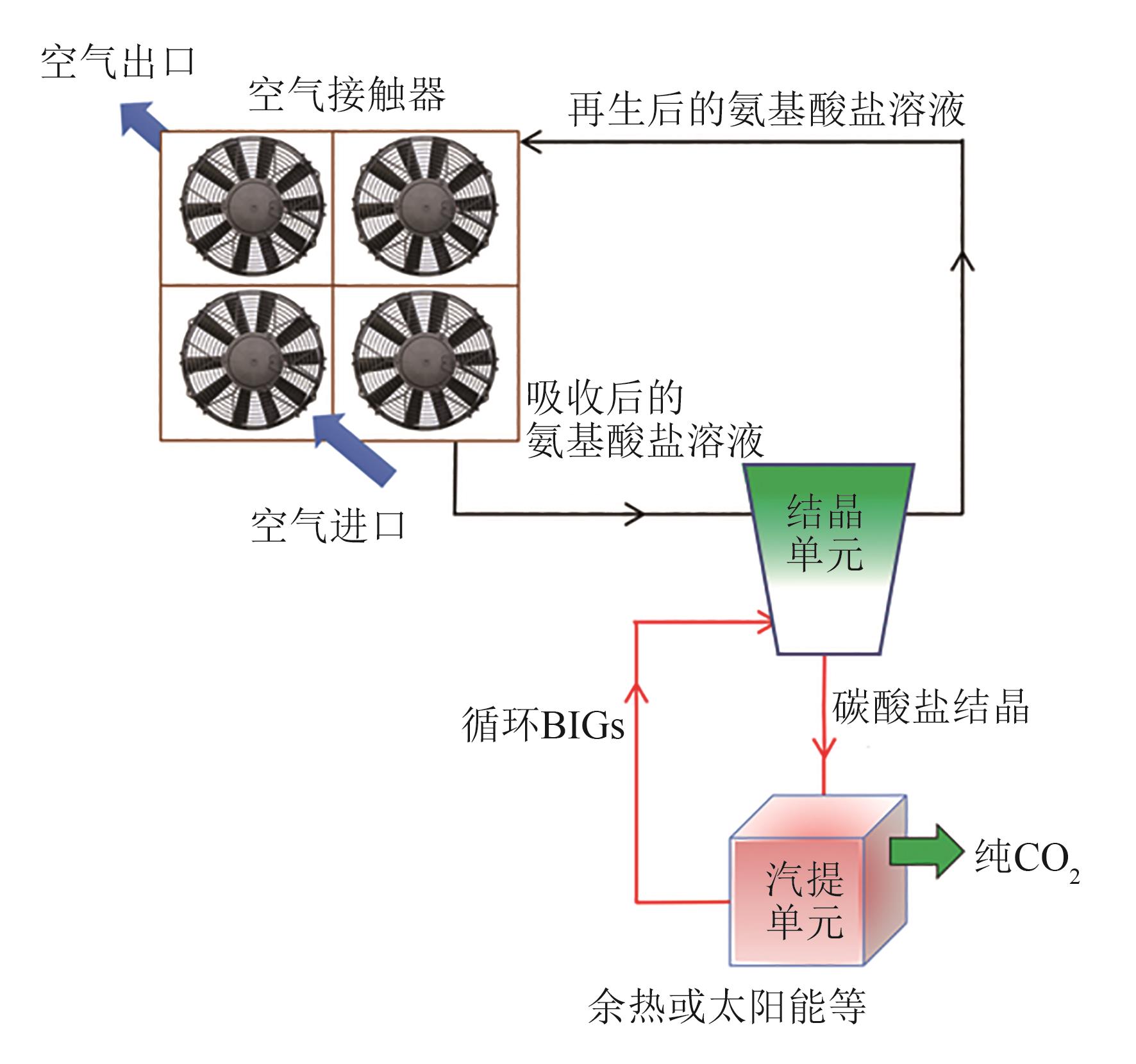
| — | 氨基酸盐溶液/m-BBIG | 60~120 | 余热 | 8.2GJ/t | — | — | — | [ |
| — | 氨基酸盐溶液/PyBIG | 80~120 | 余热 | 6.5GJ/t | — | — | — | [ |
| — | ACS | RO | 电力 | 1011~1200kWh/t | 1 | 99.8% | — | [ |
| — | ACS | MCDI | 电力 | 1072~2400kWh/t | 1 | 99.8% | — | [ |
| 1Mt/a | MI-DAC | pH改变 | 生物能 | — | — | — | 30.54 | [ |
| 捕集能力 | 吸附剂/吸收剂 | 解吸温度/℃ | 能源 | 能耗 | CO2压力/bar | CO2纯度 | 成本/USD·t-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1Mt/a | KOH | 900 | 天然气 | 8.81GJ/t | 150 | 97.1% | 94~232 | [ |
| 1Mt/a | KOH | 900 | 天然气+电力 | 5.25GJ/t+366kWh/t | 150 | 97.1% | 94~232 | [ |
| 1Mt/a | KOH | 900 | 电力 | 1535kWh/t | 1 | >97% | 186 | [ |
| 0.291t/h | MEA | 123.1 | 电力 | 1452kWh/t | 2 | — | 676 | [ |
| 0.36Mt/a | K2CO3 | 80~100 | 余热 | 7.5GJ/t+694kWh/t | — | >99% | 135~177 | [ |
| 3600t/a | K2CO3 | 80~100 | 余热 | 7.5GJ/t+694kWh/t | — | >99% | 203~244 | [ |
| — | CaO | 875 | 太阳能 | 240.9 GJ/t | — | — | — | [ |
| — | K2CO3/γ-Al2O3 | 150 | 热能+机械能 | 7.3GJ/t+0.27GJ/t | — | — | — | [ |
| 300t/a | 胺基 | 100 | 余热 | 5.4~7.2GJ/t+200~300Wh/t | — | 99.9% | 预计大规模75 | [ |
| — | 氨基聚合物 | 85~95 | 蒸汽 | 4.2~5.1GJ/t+150~260Wh/t | — | >98.5% | <113 | [ |
| — | 氨基聚合物 | 75 | 低温蒸汽 | — | — | >98.5% | ≤50 | [ |
| 140g/d | MOFs | 80,真空 | 余热 | — | — | 70%~80% | 34~350 | [ |
| 166.08t/h | 变湿吸附剂+胺液 | — | 太阳能 | 306GJ/t | — | 97% | 93.1 | [ |
| 365t/a | 变湿吸附剂 | 变湿 | 电力 | 316kWh/t | — | — | 99 (预期<30) | [ |
| — | 变湿吸附剂 | 45 | 余热 | 0.81GJ/t | — | 3% | 34.68 | [ |
| — | 氨基酸盐溶液/m-BBIG | 60~120 | 余热 | 8.2GJ/t | — | — | — | [ |
| — | 氨基酸盐溶液/PyBIG | 80~120 | 余热 | 6.5GJ/t | — | — | — | [ |
| — | ACS | RO | 电力 | 1011~1200kWh/t | 1 | 99.8% | — | [ |
| — | ACS | MCDI | 电力 | 1072~2400kWh/t | 1 | 99.8% | — | [ |
| 1Mt/a | MI-DAC | pH改变 | 生物能 | — | — | — | 30.54 | [ |
| 1 | IPCC. Climate change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change[R]. Washington: Cambridge University Press, 2021. |
| 2 | 蔡博峰, 李琦, 张贤, 等. 中国二氧化碳捕集利用与封存(CCUS) 年度报告(2021)―中国CCUS 路径研究[R]. 北京:生态环境部环境规划院, 中国科学院武汉岩土力学研究所, 中国21世纪议程管理中心,2021. |
| CAI Bofeng, LI Qi, ZHANG Xian, et al. Carbon dioxide capture, use and storage (CCUS) annual report in China (2021)—CCUS pathways in China[R]. Beijing: Chinese Academy of Environmental Planning, Institute of Rock and Soil Mechanics, Chinese Academy of Sciences, The Administrative Center for China’s Agenda 21, 2021. | |
| 3 | IEA. What would it take to limit the global temperature rise to 1.5℃?[R/OL]. Paris. [2019-11-17]. . |
| 4 | 《第四次气候变化国家评估报告》编写委员会. 第四次气候变化国家评估报告[M]. 北京: 科学出版社, 2022. |
| Committee for the preparation of the Fourth National Assessment Report on Climate Change. The fourth national assessment report on climate change[M]. Beijing: Science Press, 2022. | |
| 5 | 王鼎, 张杰, 杨伯伦, 等. 直接空气捕集CO2典型工艺分析及技术经济研究进展[J]. 煤炭科学技术,2023, 51(S1): 215-221. |
| WANG Ding, ZHANG Jie, YANG Bolun, et al. Research progress of typical process analysis and technoeconomic research on direct air capture of carbon dioxide[J]. Coal Science And Technology, 2023, 51(S1): 215-221. | |
| 6 | MCQUEEN Noah, GOMES Katherine Vaz, MCCORMICK Colin, et al. A review of direct air capture(DAC): Scaling up commercial technologies and innovating for the future[J]. Progress in Energy, 2021, 3(3): 032001. |
| 7 | BREYER Christian, FASIHI Mahdi, BAJAMUNDI Cyril, et al. Direct air capture of CO2: A key technology for ambitious climate change mitigation[J]. Joule, 2019, 3(9): 2053-2057. |
| 8 | LACKNER Klaus, ZIOCK Hans-Joachim, GRIMES Patrick. Carbon dioxide extraction from air: Is it an option?[C]//24th Annual Technical Conference on Coal Utilization & Fuel Systems. Florida: Los Alamos National Laboratory, 1999: 1-12. |
| 9 | SUTHERLAND Brandon R. Pricing CO2 direct air capture[J]. Joule, 2019, 3(7): 1571-1573. |
| 10 | 张杰, 郭伟, 张博, 等. 空气中直接捕集CO2技术研究进展[J]. 洁净煤技术, 2021, 27(2): 57-68. |
| ZHANG Jie, GUO Wei, ZHANG Bo, et al. Research progress on direct capture of CO2 from air[J]. Clean Coal Technology, 2021, 27(2): 57-68. | |
| 11 | 北京中研华泰信息技术研究院. 中国CCUS技术发展现状及未来发展前景报告2022—2027年[R]. 中国: 北京中研华泰信息技术研究院, 2022. |
| Beijing Zhongyan Huatai Information Technology Research Institute. China CCUS technology development status and future development prospects report 2022—2027[R]. China: Beijing Zhongyan Huatai Information Technology Research Institute, 2022. | |
| 12 | SODIQ Ahmed, ABDULLATIF Yasser, AISSA Brahim, et al. A review on progress made in direct air capture of CO2 [J]. Environmental Technology & Innovation, 2023, 29: 102991. |
| 13 | Climeworks. New & press[EB/OL]. . |
| 14 | Engineering Carbon. New partnership to deploy large-scale direct air capture in Norway[EB/OL]. . |
| 15 | Engineering Carbon. Engineering of world’s largest direct air capture plant begins[EB/OL]. . |
| 16 | Engineering Carbon. Occidental, 1PointFive to begin construction of world’s largest direct air capture plant in the Texas Permian Basin[EB/OL]. . |
| 17 | CHICHILNISKY G. Carbon negative power plants and their impact on environment[EB/OL]. |
| 18 | Thermostat Global. Solution[EB/OL]. . |
| 19 | IEA. Direct air capture2022, A key technology for net zero[R]. Paris: IEA, 2022. |
| 20 | 许世森. 二氧化碳捕集与利用技术研究及工程示范[R]. 中国: 中国华能集团有限公司, 2021. |
| XU Shisen. Carbon dioxide capture and utilization technology research and engineering demonstration[R]. China: China Huaneng Group, 2021. | |
| 21 | 王涛, 董昊, 侯成龙, 等. 直接空气捕集CO2吸附剂综述[J]. 浙江大学学报(工学版), 2022, 56(3): 462-475. |
| WANG Tao, DONG Hao, HOU Chenglong, et al. Review of CO2 direct air capture adsorbents[J]. Journal of Zhejiang University (Engineering Science), 2022, 56(3): 462-475. | |
| 22 | ZEMAN F, LACKNER K. Capturing carbon dioxide directly from the atmosphere[J]. World Resource Review, 2004, 16: 157-172. |
| 23 | SOCOLOW R, DESMOND M, AINES R, et al. Direct air capture of CO2 with chemicals: A technology assessment for the APS panel on public affairs[R]. America: American Physical Society, 2011. |
| 24 | FRANCESCO Sabatino, ALEXA Grimm, FAUSTO Gallucci, et al. A comparative energy and costs assessment and optimization for direct air capture technologies[J]. Joule, 2021, 5(8): 2047-2076. |
| 25 | BACIOCCHI Renato, STORTI Giuseppe, MAZZOTTI Marco. Process design and energy requirements for the capture of carbon dioxide from air[J]. Chemical Engineering and Processing: Process Intensification, 2006, 45(12): 1047-1058. |
| 26 | MASAHIRO Saito, KAZUHISA Murata. Development of high performance Cu/ZnO-based catalysts for methanol synthesis and the water-gas shift reaction[J]. Catalysis Surveys from Asia, 2004, 8(4): 285-294. |
| 27 | HODDENBAGH J, WILFING K, MILLER K, et al. Borate autocausticizing: A cost effective technology[J]. Pulp & Paper Canada, 2002, 103: 16-22. |
| 28 | MAHMOUDKHANI M, HEIDEL K R, FERREIRA J C, et al. Low energy packed tower and caustic recovery for direct capture of CO2 from air[J]. Energy Procedia, 2009, 1(1): 1535-1542. |
| 29 | 王献红. 二氧化碳捕集和利用[M]. 北京: 化学工业出版社, 2016: 88-89. |
| WANG Xianhong. CO2 capture and utilization[M]. Beijing: Chemical Industry Press, 2016: 88-89. | |
| 30 | KIM Seoni, CHOI Minjune, KANG Jin Soo, et al. Electrochemical recovery of LiOH from used CO2 adsorbents[J]. Catalysis Today, 2021, 359: 83-89. |
| 31 | SHU Qingdian, LEGRAND Louis, KUNTKE Philipp, et al. Electrochemical regeneration of spent alkaline absorbent from direct air capture[J]. Environmental Science & Technology, 2020, 54(14): 8990-8998. |
| 32 | LIU Xinmei, LU G Q, YAN Zifeng. Nanocrystalline zirconia as catalyst support in methanol synthesis[J]. Applied Catalysis A: General, 2005, 279(1/2): 241-245. |
| 33 | 郭晓明. 二氧化碳加氢合成甲醇铜基催化剂的研究[D]. 上海: 华东理工大学, 2011. |
| GUO Xiaoming. Study of Cu-based catalysts for methanol synthesis by CO2 hydrogenation[D]. Shanghai: East China University of Science and Technology, 2011. | |
| 34 | ZEMAN Frank. Experimental results for capturing CO2 from the atmosphere[J]. AIChE Journal, 2008, 54(5): 1396-1399. |
| 35 | KEITH David W, HOLMES Geoffrey, ANGELO David ST, et al. A process for capturing CO2 from the atmosphere[J]. Joule, 2018, 2(10): 2179. |
| 36 | ROCHELLE Gary T. Amine scrubbing for CO2 capture[J]. Science, 2009, 325(5948): 1652-1654. |
| 37 | LACKNER K S. Capture of carbon dioxide from ambient air[J]. The European Physical Journal Special Topics, 2009, 176(1): 93-106. |
| 38 | VELTMAN Karin, SINGH Bhawna, HERTWICH Edgar G. Human and environmental impact assessment of postcombustion CO2 capture focusing on emissions from amine-based scrubbing solvents to air[J]. Environmental Science & Technology, 2010, 44(4): 1496-1502. |
| 39 | LE MOULLEC Yann, NEVEUX Thibaut, AZKI Adam AL, et al. Process modifications for solvent-based post-combustion CO2 capture[J]. International Journal of Greenhouse Gas Control, 2014, 31: 96-112. |
| 40 | BARZAGLI F, MANI F. Direct CO2 air capture with aqueous 2-(ethylamino)ethanol and 2-(2-aminoethoxy)ethanol: 13C NMR speciation of the absorbed solutions and study of the sorbent regeneration improved by a transition metal oxide catalyst[J]. Inorganica Chimica Acta, 2021, 518: 120256. |
| 41 | KIANI Ali, JIANG Kaiqi, FERON Paul. Techno-economic assessment for CO2 capture from air using a conventional liquid-based absorption process[J]. Frontiers in Energy Research, 2020, 8: 92. |
| 42 | SEIPP Charles A, WILLIAMS Neil J, KIDDER Michelle K, et al. CO2 capture from ambient air by crystallization with a guanidine sorbent[J]. Angewandte Chemie International Edition, 2017, 56(4): 1042-1045. |
| 43 | CUSTELCEAN Radu, WILLIAMS Neil J, GARRABRANT Kathleen A, et al. Direct air capture of CO2 with aqueous amino acids and solid bis-iminoguanidines (BIGs)[J]. Industrial & Engineering Chemistry Research, 2019, 58(51): 23338-23346. |
| 44 | BRETHOMÉ Flavien M, WILLIAMS Neil J, SEIPP Charles A, et al. Direct air capture of CO2 via aqueous-phase absorption and crystalline-phase release using concentrated solar power[J]. Nature Energy, 2018, 3(7): 553-559. |
| 45 | CUSTELCEAN Radu, WILLIAMS Neil J, WANG Xiaoping, et al. Dialing in direct air capture of CO2 by crystal engineering of bisiminoguanidines[J]. ChemSusChem, 2020, 13(23): 6381-6390. |
| 46 | RINBERG Anatoly, BERGMAN Andrew M, SCHRAG Daniel P, et al. Alkalinity concentration swing for direct air capture of carbon dioxide[J]. ChemSusChem, 2021, 14(20): 4439-4453. |
| 47 | ELIMELECH Menachem, PHILLIP William A. The future of seawater desalination: Energy, technology, and the environment[J]. Science, 2011, 333(6043): 712-717. |
| 48 | SUSS M E, PORADA S, SUN X, et al. Water desalination via capacitive deionization: What is it and what can we expect from it?[J]. Energy & Environmental Science, 2015, 8(8): 2296-2319. |
| 49 | Sajjad AL-AMSHAWEE, BIN MOHD YUNUS Mohd Yusri, AZODDEIN Abdul Aziz Mohd, et al. Electrodialysis desalination for water and wastewater: A review[J]. Chemical Engineering Journal, 2020, 380: 122231. |
| 50 | NIKULSHINA V, STEINFELD A. CO2 capture from air via CaO-carbonation using a solar-driven fluidized bed reactor—Effect of temperature and water vapor concentration[J]. Chemical Engineering Journal, 2009, 155(3): 867-873. |
| 51 | NIKULSHINA V, GEBALD C, STEINFELD A. CO2 capture from atmospheric air via consecutive CaO-carbonation and CaCO3-calcination cycles in a fluidized-bed solar reactor[J]. Chemical Engineering Journal, 2009, 146(2): 244-248. |
| 52 | NIKULSHINA V, GÁLVEZ M E, STEINFELD A. Kinetic analysis of the carbonation reactions for the capture of CO2 from air via the Ca(OH)2-CaCO3-CaO solar thermochemical cycle[J]. Chemical Engineering Journal, 2007, 129(1/2/3): 75-83. |
| 53 | NIKULSHINA V, AYESA N, GÁLVEZ M E, et al. Feasibility of Na-based thermochemical cycles for the capture of CO2 from air—Thermodynamic and thermogravimetric analyses[J]. Chemical Engineering Journal, 2008, 140(1/2/3): 62-70. |
| 54 | STOLAROFF Joshuah K, LOWRY Gregory V, KEITH David W. Using CaO- and MgO-rich industrial waste streams for carbon sequestration[J]. Energy Conversion and Management, 2005, 46(5): 687-699. |
| 55 | COLOMBO G. Study of CO2 sorbents for extravehicular activity[R]. America: NASA, 1973 |
| 56 | CAMPBELL J S. Decomposition of carbonates in capture of carbon dioxide from ambient air[D]. Vancouver: University of British Columbia, 2019. |
| 57 | RANJAN Manya, HERZOG Howard J. Feasibility of air capture[J]. Energy Procedia, 2011, 4: 2869-2876. |
| 58 | VESELOVSKAYA Janna V, DEREVSCHIKOV Vladimir S, KARDASH Tatyana Yu, et al. Direct CO2 capture from ambient air using K2CO3/Al2O3 composite sorbent[J]. International Journal of Greenhouse Gas Control, 2013, 17: 332-340. |
| 59 | Jacek PRZEPIÓRSKI, Adam CZYŻEWSKI, PIETRZAK Robert, et al. MgO/CaO-loaded activated carbon for carbon dioxide capture: Practical aspects of use[J]. Industrial & Engineering Chemistry Research, 2013, 52(20): 6669-6677. |
| 60 | BALI S, SAKWA-NOVAK Miles A, JONES Christopher W. Potassium incorporated alumina based CO2 capture sorbents: Comparison with supported amine sorbents under ultra-dilute capture conditions[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015, 486: 78-85. |
| 61 | DEREVSCHIKOV V, VESELOVSKAYA J, KARDASH T, et al. Direct CO2 capture from ambient air using K2CO3/Y2O3 composite sorbent[J]. Fuel, 2014, 127: 212-218. |
| 62 | ZHAO Chuanwen, GUO Yafei, LI Changhai, et al. Carbonation behavior of K2CO3/AC in low reaction temperature and CO2 concentration[J]. Chemical Engineering Journal, 2014, 254: 524-530. |
| 63 | ZHAO Chuanwen, GUO Yafei, LI Changhai, et al. Removal of low concentration CO2 at ambient temperature using several potassium-based sorbents[J]. Applied Energy, 2014, 124: 241-247. |
| 64 | VESELOVSKAYA Janna V, DEREVSCHIKOV Vladimir S, SHALYGIN Anton S, et al. K2CO3-containing composite sorbents based on a ZrO2 aerogel for reversible CO2 capture from ambient air[J]. Microporous and Mesoporous Materials, 2021, 310: 110624. |
| 65 | SANZ-PÉREZ Eloy S, MURDOCK Christopher R, DIDAS Stephanie A, et al. Direct capture of CO2 from ambient air[J]. Chemical Reviews, 2016, 116(19): 11840-11876. |
| 66 | CAPLOW Michael. Kinetics of carbamate formation and breakdown[J]. Journal of the American Chemical Society, 1968, 90(24): 6795-6803. |
| 67 | DANCKWERTS P V. The reaction of CO2 with ethanolamines[J]. Chemical Engineering Science, 1979, 34(4): 443-446. |
| 68 | YANG Zhenzhen, HE Liangnian, ZHAO Yanan, et al. CO2 capture and activation by superbase/polyethylene glycol and its subsequent conversion[J]. Energy & Environmental Science, 2011, 4(10): 3971-3975. |
| 69 | ZHU Xuancan, GE Tianshu, YANG Fan, et al. Design of steam-assisted temperature vacuum-swing adsorption processes for efficient CO2 capture from ambient air[J]. Renewable and Sustainable Energy Reviews, 2021, 137: 110651. |
| 70 | SINHA Anshuman, DARUNTE Lalit A, JONES Christopher W, et al. Systems design and economic analysis of direct air capture of CO2 through temperature vacuum swing adsorption using MIL-101(Cr)-PEI-800 and mmen-Mg2(dobpdc) MOF adsorbents[J]. Industrial & Engineering Chemistry Research, 2017, 56(3): 750-764. |
| 71 | LIN Zhifeng, WEI Jianwen, GENG Linlin, et al. An amine double functionalized composite strategy for CO2 adsorbent preparation using a ZSM-5/KIT-6 composite as a support[J]. Energy Technology, 2018, 6(9): 1618-1626. |
| 72 | WILFONG Walter Christopher, KAIL Brian W, JONES Christopher W, et al. Spectroscopic investigation of the mechanisms responsible for the superior stability of hybrid class 1/class 2 CO2 sorbents: A new class 4 category[J]. ACS Applied Materials & Interfaces, 2016, 8(20): 12780-12791. |
| 73 | 孔祥如, 张肖阳, 孙鹏翔, 等. 直接空气捕碳固体多孔材料的研究进展[J]. 化工进展, 2023, 42(3): 1471-1483. |
| KONG Xiangru, ZHANG Xiaoyang, SUN Pengxiang, et al. Research progress of solid porous materials for direct CO2 capture from air[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1471-1483. | |
| 74 | MIAO Yihe, HE Zhijun, ZHU Xuancan, et al. Operating temperatures affect direct air capture of CO2 in polyamine-loaded mesoporous silica[J]. Chemical Engineering Journal, 2021, 426: 131875. |
| 75 | LIU Faqian, WANG Lei, HUANG Zhaoge, et al. Amine-tethered adsorbents based on three-dimensional macroporous silica for CO2 capture from simulated flue gas and air[J]. ACS Applied Materials & Interfaces, 2014, 6(6): 4371-4381. |
| 76 | WANG Jitong, HUANG Haihong, WANG Mei, et al. Direct capture of low-concentration CO2 on mesoporous carbon-supported solid amine adsorbents at ambient temperature[J]. Industrial & Engineering Chemistry Research, 2015, 54(19): 5319-5327. |
| 77 | KELLER Laura, Burkhard OHS, LENHART Jelena, et al. High capacity polyethylenimine impregnated microtubes made of carbon nanotubes for CO2 capture[J]. Carbon, 2018, 126: 338-345. |
| 78 | GEBALD Christoph, WURZBACHER Jan Andre, TINGAUT Philippe, et al. Amine-based nanofibrillated cellulose as adsorbent for CO2 capture from air[J]. Environmental Science & Technology, 2011, 45(20): 9101-9108. |
| 79 | ZHAO Meng, XIAO Jiewen, GAO Wanlin, et al. Defect-rich Mg-Al MMOs supported TEPA with enhanced charge transfer for highly efficient and stable direct air capture[J]. Journal of Energy Chemistry, 2022, 68: 401-410. |
| 80 | ZHU Xuancan, Meng LYU, GE Tianshu, et al. Modified layered double hydroxides for efficient and reversible carbon dioxide capture from air[J]. Cell Reports Physical Science, 2021, 2(7): 100484. |
| 81 | KULKARNI Vaishnavi, PANDA Debashis, SINGH Sanjay Kumar. Direct air capture of CO2 over amine-modified hierarchical silica[J]. Industrial & Engineering Chemistry Research, 2023, 62(8): 3800-3811. |
| 82 | LIAO Peiqin, CHEN Xunwei, LIU Siyang, et al. Putting an ultrahigh concentration of amine groups into a metal-organic framework for CO2 capture at low pressures[J]. Chemical Science, 2016, 7(10): 6528-6533. |
| 83 | ZHANG Jianxin, ZHAO Qing, WANG Shidi, et al. Direct capture of low concentration CO2 using tetraethylenepentamine-grafted polyacrylonitrile hollow fibers[J]. Separation and Purification Technology, 2022, 287: 120562. |
| 84 | 宋珂琛, 崔希利, 邢华斌. 二氧化碳直接空气捕集材料与技术研究进展[J]. 化工进展, 2022, 41(3): 1152-1162. |
| SONG Kechen, CUI Xili, XING Huabin. Progress on direct air capture of carbon dioxide[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1152-1162. | |
| 85 | QI Luming, HAN Yu, BAI Gaozhi, et al. Role of brush-like additives in CO2 adsorbents for the enhancement of amine efficiency[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106709. |
| 86 | WANG Jitong, LONG Donghui, ZHOU Huanhuan, et al. Surfactant promoted solid amine sorbents for CO2 capture[J]. Energy & Environmental Science, 2012, 5(2): 5742-5749. |
| 87 | Aliakbar HEYDARI-GORJI, BELMABKHOUT Youssef, SAYARI Abdelhamid. Polyethylenimine-impregnated mesoporous silica: Effect of amine loading and surface alkyl chains on CO2 adsorption[J]. Langmuir, 2011, 27(20): 12411-12416. |
| 88 | METH S, GOEPPERT A, SURYA PRAKASH G K, et al. Silica nanoparticles as supports for regenerable CO2 sorbents[J]. Energy & Fuels, 2012, 26(5): 3082-3090. |
| 89 | SAKWA-NOVAK Miles A, TAN Shuai, JONES Christopher W. Role of additives in composite PEI/oxide CO2 adsorbents: Enhancement in the amine efficiency of supported PEI by PEG in CO2 capture from simulated ambient air[J]. ACS Applied Materials & Interfaces, 2015, 7(44): 24748-24759. |
| 90 | XU Xiaochun, SONG Chunshan, ANDRÉSEN John M, et al. Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-modified mesoporous molecular sieve MCM-41[J]. Microporous and Mesoporous Materials, 2003, 62(1/2): 29-45. |
| 91 | BELMABKHOUT Youssef, Rodrigo SERNA-GUERRERO, SAYARI Abdelhamid. Adsorption of CO2-containing gas mixtures over amine-bearing pore-expanded MCM-41 silica: Application for gas purification[J]. Industrial & Engineering Chemistry Research, 2010, 49(1): 359-365. |
| 92 | GOEPPERT Alain, CZAUN Miklos, Robert B MAY, et al. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent[J]. Journal of the American Chemical Society, 2011, 133(50): 20164-20167. |
| 93 | GUTKNECHT Valentin, SNÆBJÖRNSDÓTTIR Sandra Ósk, Bergur SIGFÚSSON, et al. Creating a carbon dioxide removal solution by combining rapid mineralization of CO2 with direct air capture[J]. Energy Procedia, 2018, 146: 129-134. |
| 94 | CHRISTOPH Beuttler, LOUISE Charles, Wurzbacher JAN. The role of direct air capture in mitigation of anthropogenic greenhouse gas emissions[J]. Frontiers in Climate, 2019, 1: 1-7. |
| 95 | GRACIELA C. Carbon negative power plants and their impact on environment[R]. Argentina: Earth Dialogues, 2018. |
| 96 | SHEKHAH Osama, BELMABKHOUT Youssef, CHEN Zhijie, et al. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture[J]. Nature Communications, 2014, 5: 4228. |
| 97 | SHEKHAH Osama, BELMABKHOUT Youssef, ADIL Karim, et al. A facile solvent-free synthesis route for the assembly of a highly CO2 selective and H2S tolerant NiSIFSIX metal-organic framework[J]. Chemical Communications, 2015, 51(71): 13595-13598. |
| 98 | ZHANG Zhaoqiang, DING Qi, CUI Jiyu, et al. High and selective capture of low-concentration CO2 with an anion-functionalized ultramicroporous metal-organic framework[J]. Science China Materials, 2021, 64(3): 691-697. |
| 99 | BHATT Prashant M, BELMABKHOUT Youssef, CADIAU Amandine, et al. A fine-tuned fluorinated MOF addresses the needs for trace CO2 removal and air capture using physisorption[J]. Journal of the American Chemical Society, 2016, 138(29): 9301-9307. |
| 100 | DING Meili, FLAIG Robinson W, JIANG Hailong, et al. Carbon capture and conversion using metal-organic frameworks and MOF-based materials[J]. Chemical Society Reviews, 2019, 48(10): 2783-2828. |
| 101 | SADIQ Muhammad Munir, BATTEN Michael P, MULET Xavier, et al. A pilot-scale demonstration of mobile direct air capture using metal-organic frameworks[J]. Advanced Sustainable Systems, 2020, 4(12): 2000101. |
| 102 | SADIQ M M, BATTEN M, THORNTON A W, et al. Adsorption and desorption appratus: WO2020113281[P]. 2020-6-11. |
| 103 | WANG Tao, LACKNER Klaus S, WRIGHT Allen. Moisture swing sorbent for carbon dioxide capture from ambient air[J]. Environmental Science & Technology, 2011, 45(15): 6670-6675. |
| 104 | QUINN R, APPLEBY J B, PEZ G P. Salt hydrates: New reversible absorbents for carbon dioxide[J]. Journal of the American Chemical Society, 1995, 117(1): 329-335. |
| 105 | WANG Tao, GE Kun, CHEN Kexian, et al. Theoretical studies on CO2 capture behavior of quaternary ammonium-based polymeric ionic liquids[J]. Physical Chemistry Chemical Physics, 2016, 18(18): 13084-13091. |
| 106 | WANG Tao, HOU Chenglong, GE Kun, et al. Spontaneous cooling absorption of CO2 by a polymeric ionic liquid for direct air capture[J]. The Journal of Physical Chemistry Letters, 2017, 8(17): 3986-3990. |
| 107 | WANG Tao, LIU Jun, HUANG Hao, et al. Preparation and kinetics of a heterogeneous sorbent for CO2 capture from the atmosphere[J]. Chemical Engineering Journal, 2016, 284: 679-686. |
| 108 | SHI Xiaoyang, LI Qibin, WANG Tao, et al. Kinetic analysis of an anion exchange absorbent for CO2 capture from ambient air[J]. PLoS One, 2017, 12(6): e0179828. |
| 109 | WANG Tao, LIU Jun, LACKNER Klaus S, et al. Characterization of kinetic limitations to atmospheric CO2 capture by solid sorbent[J]. Greenhouse Gases: Science and Technology, 2016, 6(1): 138-149. |
| 110 | HE Hongkun, LI Wenwen, ZHONG Mingjiang, et al. Reversible CO2 capture with porous polymers using the humidity swing[J]. Energy & Environmental Science, 2013, 6(2): 488-493. |
| 111 | WANG Tao, WANG Xinru, HOU Chenglong, et al. Quaternary functionalized mesoporous adsorbents for ultra-high kinetics of CO2 capture from air[J]. Scientific Reports, 2020, 10: 21429. |
| 112 | 吴禹松. 用于空气二氧化碳捕集的多孔树脂吸附剂成型及性能研究[D]. 杭州: 浙江大学, 2020. |
| WU Yusong. Research on formation and performance of porous resin adsorbent for direct air capture of CO2 [D]. Hangzhou: Zhejiang University, 2020. | |
| 113 | SONG Juzheng, LIU Jie, ZHAO Wei, et al. Quaternized chitosan/PVA aerogels for reversible CO2 capture from ambient air[J]. Industrial & Engineering Chemistry Research, 2018, 57(14): 4941-4948. |
| 114 | 徐锶瑶, 侯成龙, 王涛. 季铵型变湿再生材料CO2吸附热量迁移研究[J]. 能源工程, 2021(1): 54-62, 88. |
| XU Siyao, HOU Chenglong, WANG Tao. The heat transfer of quaternary ammonium resin materials in the adsorption process[J]. Energy Engineering, 2021(1): 54-62, 88. | |
| 115 | HOU Chenglong, WU Yusong, WANG Tao, et al. Preparation of quaternized bamboo cellulose and its implication in direct air capture of CO2 [J]. Energy & Fuels, 2019, 33(3): 1745-1752. |
| 116 | SHI Xiaoyang, XIAO Hang, AZARABADI Habib, et al. Sorbents for the direct capture of CO2 from ambient air[J]. Angewandte Chemie International Edition, 2020, 59(18): 6984-7006. |
| 117 | HOU Chenglong, WU Yusong, JIAO Youzhou, et al. Integrated direct air capture and CO2 utilization of gas fertilizer based on moisture swing adsorption[J]. Journal of Zhejiang University:Science A, 2017, 18(10): 819-830. |
| 118 | DUYAR Melis S, Martha A Arellano TREVIÑO, FARRAUTO Robert J. Dual function materials for CO2 capture and conversion using renewable H2 [J]. Applied Catalysis B: Environmental, 2015, 168/169: 370-376. |
| 119 | MELO BRAVO Paulina, DEBECKER Damien P. Combining CO2 capture and catalytic conversion to methane[J]. Waste Disposal & Sustainable Energy, 2019, 1(1): 53-65. |
| 120 | Chae JEONG-POTTER, ABDALLAH Monica, SANDERSON Cory, et al. Dual function materials (Ru+Na2O/Al2O3) for direct air capture of CO2 and in situ catalytic methanation: The impact of realistic ambient conditions[J]. Applied Catalysis B: Environmental, 2022, 307: 120990. |
| 121 | 王焕君, 刘牛, 郑棹方, 等. 直接空气捕碳材料研究进展[J]. 发电技术, 2022, 43(4): 533-543. |
| WANG Huanjun, LIU Niu, ZHENG Zhaofang, et al. Research progress of materials for direct capture of CO2 from ambient air[J]. Power Generation Technology, 2022, 43(4): 533-543. | |
| 122 | VESELOVSKAYA Janna V, PARUNIN Pavel D, NETSKINA Olga V, et al. A novel process for renewable methane production: Combining direct air capture by K2CO3/alumina sorbent with CO2 methanation over Ru/alumina catalyst[J]. Topics in Catalysis, 2018, 61(15): 1528-1536. |
| 123 | VESELOVSKAYA Janna V, LYSIKOV Anton I, NETSKINA Olga V, et al. K2CO3-containing composite sorbents based on thermally modified alumina: Synthesis, properties, and potential application in a direct air capture/methanation process[J]. Industrial & Engineering Chemistry Research, 2020, 59(15): 7130-7139. |
| 124 | QIAO Yuanting, BAILEY Josh J, HUANG Qi, et al. Potential photo-switching sorbents for CO2 capture—A review[J]. Renewable and Sustainable Energy Reviews, 2022, 158: 112079. |
| 125 | PRASETYA Nicholaus, LADEWIG Bradley P. Dynamic photo-switching in light-responsive JUC-62 for CO2 capture[J]. Scientific Reports, 2017, 7: 13355. |
| 126 | WILM Lukas F B, Mowpriya DAS, Daniel JANSSEN-MÜLLER, et al. Photoswitchable nitrogen superbases: Using light for reversible carbon dioxide capture[J]. Angewandte Chemie International Edition, 2022, 61(3): e202112344. |
| 127 | 孙静静, 吴仇荣, 翁文强, 等. 调控强活性位点的智能光响应CO2吸附剂[J]. 化学学报, 2020, 78(10): 1082-1088. |
| SUN Jingjing, WU Qiurong, WENG Wenqiang, et al. Smart light-responsive CO2 adsorbents for regulating strong active sites[J]. Acta Chimica Sinica, 2020, 78(10): 1082-1088. | |
| 128 | 李倩, 王钰, 李睿, 等. 光响应CO2吸附剂的制备及CO2吸附脱附性能的研究[J]. 石河子大学学报(自然科学版), 2021, 39(1): 1-7. |
| LI Qian, WANG Yu, LI Rui, et al. Preparation of photoresponsive CO2 adsorbent and study on CO2 adsorption desorption performance[J]. Journal of Shihezi University (Natural Science), 2021, 39(1): 1-7. | |
| 129 | SU Jing, TENG Hui Henry, WAN Xiang, et al. Direct air capture of CO2 through carbonate alkalinity generated by phytoplankton nitrate assimilation[J]. International Journal of Environmental Research and Public Health, 2022, 20(1): 550. |
| 130 | ALEXANDER Caskie. Technical, policy and stakeholder analysis of direct air capture[D]. Nederland: Delft University of Technology, 2020. |
| 131 | FASIHI Mahdi, EFIMOVA Olga, BREYER Christian. Techno-economic assessment of CO2 direct air capture plants[J]. Journal of Cleaner Production, 2019, 224: 957-980. |
| [1] | GUO Xiaodong, MAO Yujiao, LIU Xiangyang, QIU Li, YU Feng, YAN Xiaoliang. Effect of oxygen vacancies in Ni/Sm2O3-CeO2/Al2O3 catalyst on CO2 methanation at low temperature [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1840-1850. |
| [2] | YANG Dongxiao, XIONG Qizhao, WANG Yi, CHEN Yang, LI Libo, LI Jinping. Progress in the preparation of hierarchically porous MOF and applications in adsorption and separation [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1882-1896. |
| [3] | WANG Debin, LIN Mengyu, YANG Xue, DONG Dianquan. Preparation and adsorption properties of zinc-doped titanium-based cesium ion sieves [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1953-1961. |
| [4] | WANG Kai, YE Dingding, ZHU Xun, YANG Yang, CHEN Rong, LIAO Qiang. Performance of electrochemical reduction of CO2 by superaerophilic copper foam electrode with nanowires [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1232-1240. |
| [5] | LIU Fangwang, HAN Yi, ZHANG Jiajia, BU Honghong, WANG Xingpeng, YU Chuanfeng, LIU Mengshuai. Research advance of heterogeneous catalytic system for the coupling between CO2 and epoxide into propylene carbonate [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1252-1265. |
| [6] | DONG Xiaohan, TIAN Yue, SU Yi. Study on the preparation of composite adsorbent with titanium-containing blast furnace slag and chromium adsorption performance [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1552-1564. |
| [7] | CHEN Linlin, YU Fei, Ma JIE. Preparation of wood-based cellulose/graphene separation membrane and pollutant separation performance [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1584-1592. |
| [8] | PENG Cheng, XU Yilin, SHI Yujing, ZHANG Wen, LI Yutao, WANG Haoran, ZHANG Wei, ZHAN Xiuping. Research progress on the biochar modification and its remediation of herbicide-contaminated water and soil [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 1069-1081. |
| [9] | GUO Yingchun, LIANG Xiaoyi. Effect of citric acid modification on the spherical activated carbon's ammonia adsorption performance [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 1082-1088. |
| [10] | MIAO Feng, XU Chuanlong, LI Jian, ZHANG Biao, HAN Shaopeng, TANG Guanghua. Online calibration of the wavelength of spectrometer based on SO2 absorption spectrum [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 818-822. |
| [11] | HUANG Sheng, YANG Zhenli, LI Zhenyu. Analysis of optimization path of developing China's hydrogen industry [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 882-893. |
| [12] | YU Xiaoxiao, CHAO Yanhong, LIU Haiyan, ZHU Wenshuai, LIU Zhichang. Enhanced photoelectric properties and photocatalytic CO2 conversion by D-A conjugated polymerization [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 292-301. |
| [13] | CHEN Le, CHONG Hailing, ZHANG Zhihui, HE Mingyang, CHEN Qun. Synthesis of Cu-BTC modified by CTAB and its adsorption and separation of xylene isomers [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 455-464. |
| [14] | YANG Mengru, PENG Qin, CHANG Yulong, QIU Shuxing, ZHANG Jianbo, JIANG Xia. Research progress of carbon emission reduction technology with biochar replacing pulverized coal/coke for blast furnace ironmaking [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 490-500. |
| [15] | CHENG Haolin, NIAN Yao, HAN You. Progress in the mechanism of CH4 and CO2co-conversion reactions [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 60-75. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||