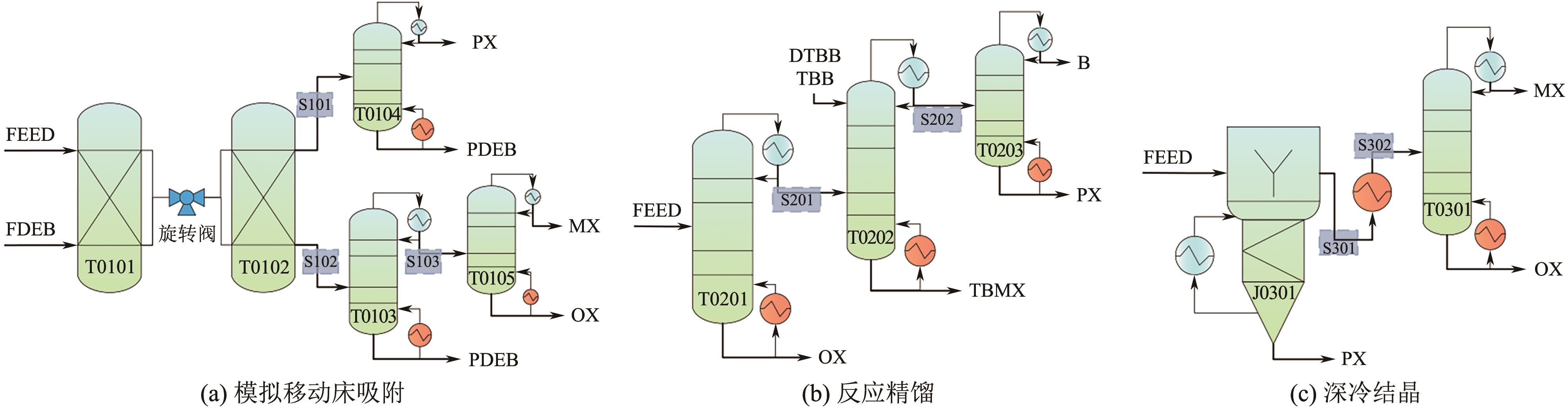
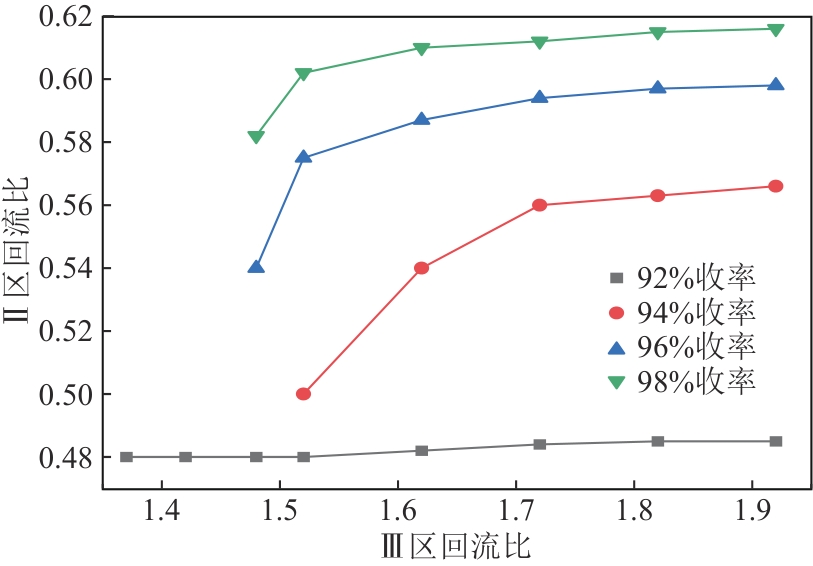
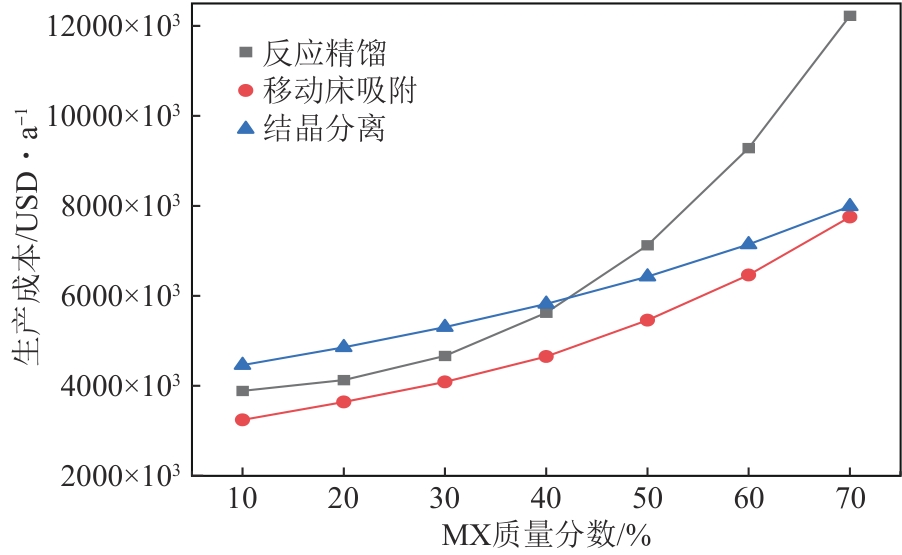
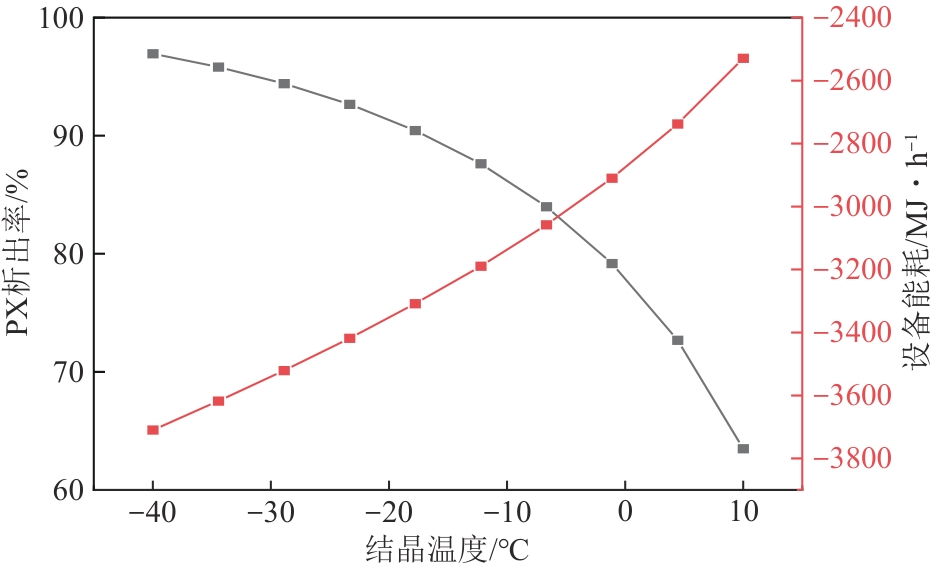
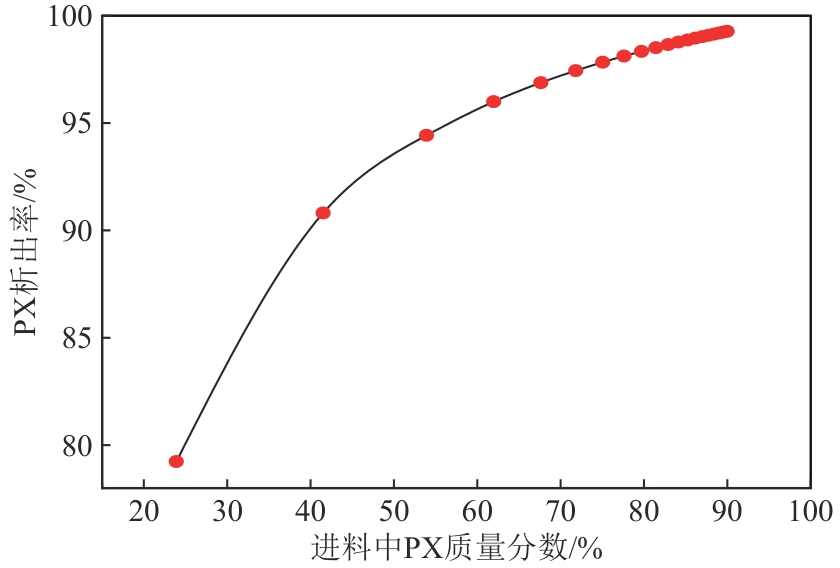
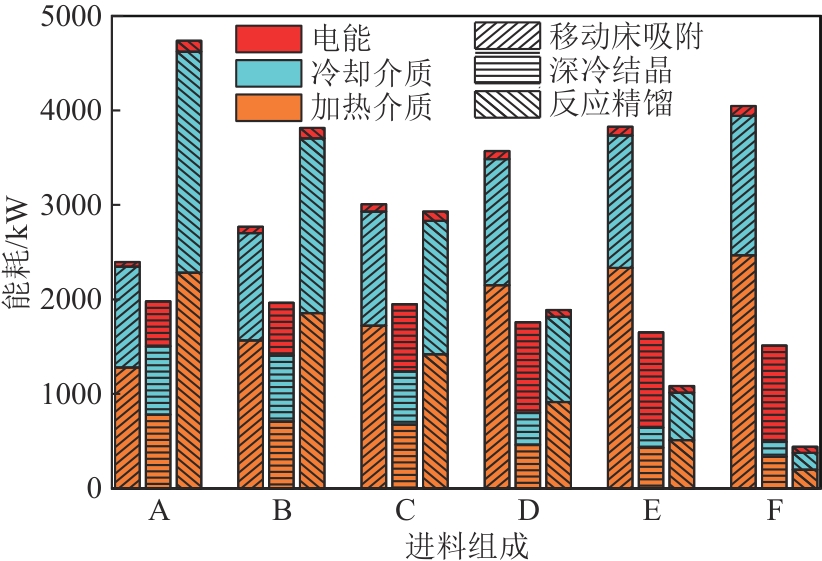
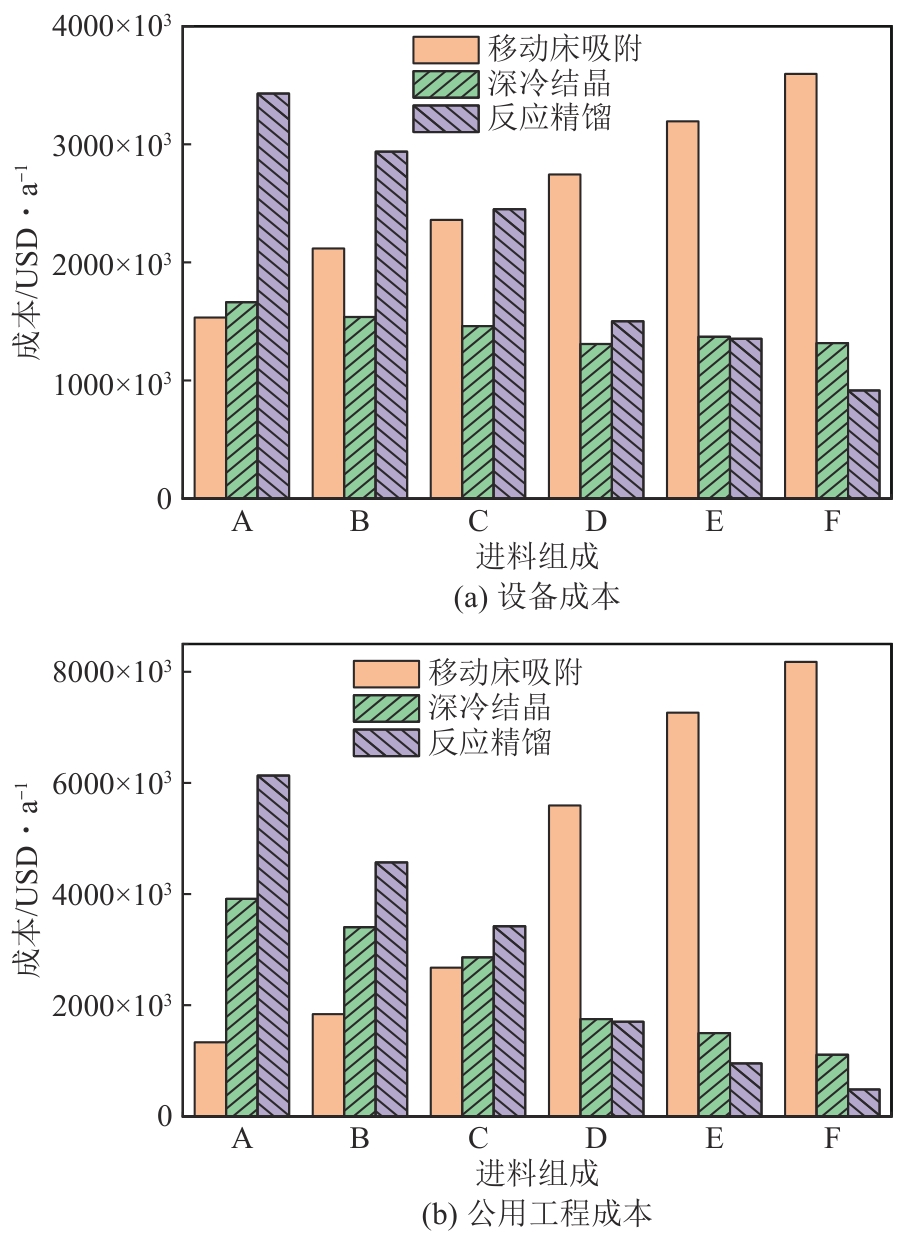
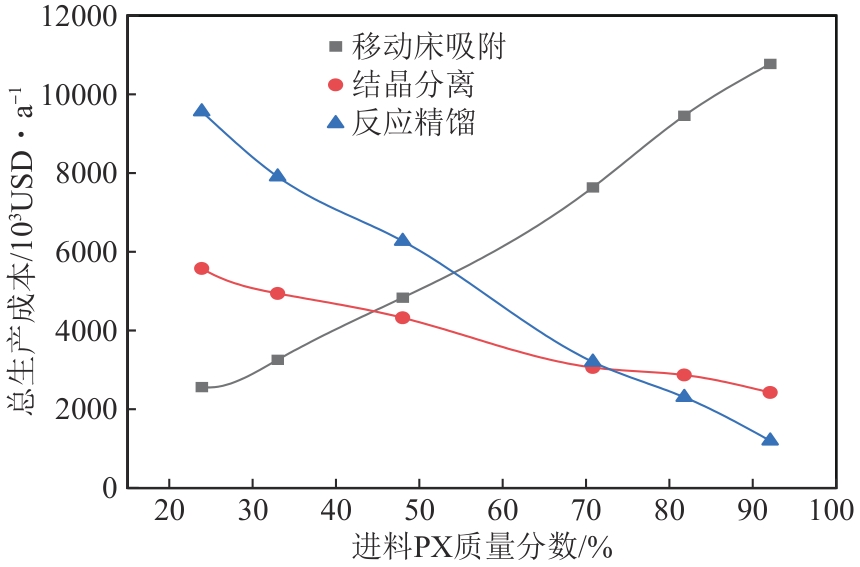
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (8): 4732-4740.DOI: 10.16085/j.issn.1000-6613.2025-0154
• Process systems modeling and simulation • Previous Articles
Technical-economic evaluation for different separation strategies of xylene isomers
YANG Yong1,2( ), ZHANG Zhao1, WANG Dongliang1,2, ZHOU Huairong1,2, ZHAO Zihao1, LI Yukun1
), ZHANG Zhao1, WANG Dongliang1,2, ZHOU Huairong1,2, ZHAO Zihao1, LI Yukun1
- 1.School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
2.Key Laboratory of Low Carbon Energy and Chemical Engineering of Gansu Province, Lanzhou 730050, Gansu, China
-
Received:2025-02-07Revised:2025-05-12Online:2025-09-08Published:2025-08-25 -
Contact:YANG Yong
二甲苯异构体不同分离策略的技术经济评价
杨勇1,2( ), 张钊1, 王东亮1,2, 周怀荣1,2, 赵子豪1, 李煜坤1
), 张钊1, 王东亮1,2, 周怀荣1,2, 赵子豪1, 李煜坤1
- 1.兰州理工大学石油化工学院,甘肃 兰州 730050
2.甘肃省低碳能源化工重点实验室,甘肃 兰州 730050
-
通讯作者:杨勇 -
作者简介:杨勇(1986—),男,博士,副教授,研究方向为化工系统工程。E-mail:yangy@lut.edu.cn。 -
基金资助:国家自然科学基金(22468030);甘肃省科技重大专项(23ZDGF002)
CLC Number:
Cite this article
YANG Yong, ZHANG Zhao, WANG Dongliang, ZHOU Huairong, ZHAO Zihao, LI Yukun. Technical-economic evaluation for different separation strategies of xylene isomers[J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4732-4740.
杨勇, 张钊, 王东亮, 周怀荣, 赵子豪, 李煜坤. 二甲苯异构体不同分离策略的技术经济评价[J]. 化工进展, 2025, 44(8): 4732-4740.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2025-0154
| 类别 | 对二甲苯 | 间二甲苯 | 邻二甲苯 | 催化剂 | 参考文献 |
|---|---|---|---|---|---|
| A | 23.89 | 53.26 | 22.84 | [ | |
| B | 33.00 | 48.00 | 19.00 | ZnZrO x /4Z5 | [ |
| C | 48.00 | 26.85 | 25.15 | ZnZrO x /2Z5 | [ |
| D | 70.81 | 20.10 | 9.06 | ZnZrO x /2Z5 | [ |
| E | 81.79 | 12.26 | 5.95 | ZrCuO0.1-Z50.5(25)-Si(2) | [ |
| F | 92.09 | 3.98 | 3.93 | ZSM-5 | [ |
| 类别 | 对二甲苯 | 间二甲苯 | 邻二甲苯 | 催化剂 | 参考文献 |
|---|---|---|---|---|---|
| A | 23.89 | 53.26 | 22.84 | [ | |
| B | 33.00 | 48.00 | 19.00 | ZnZrO x /4Z5 | [ |
| C | 48.00 | 26.85 | 25.15 | ZnZrO x /2Z5 | [ |
| D | 70.81 | 20.10 | 9.06 | ZnZrO x /2Z5 | [ |
| E | 81.79 | 12.26 | 5.95 | ZrCuO0.1-Z50.5(25)-Si(2) | [ |
| F | 92.09 | 3.98 | 3.93 | ZSM-5 | [ |
| SMB结构参数 | 吸附操作条件 | 吸附模型参数 | ||
|---|---|---|---|---|
| 吸附剂参数 | 吸附平衡常数 | 最大吸附量 | ||
| HSMB=122.7cm | t=70s | ρ=876kg/m3 | KPX=1.0750 | qmPX=0.168kg/kg |
| DSMB=600cm | T=177℃ | ε=0.39 | KOX=0.2850 | qmOX=0.168kg/kg |
| 床层数分配7-9-5-3 | p=0.88MPa | dp=0.56mm | KMX=0.2645 | qmMX=0.168kg/kg |
| Pe=2000 | KPDEB=1.3125 | qmPDEB=0.138kg/kg | ||
| KL=9min-1 | ||||
| SMB结构参数 | 吸附操作条件 | 吸附模型参数 | ||
|---|---|---|---|---|
| 吸附剂参数 | 吸附平衡常数 | 最大吸附量 | ||
| HSMB=122.7cm | t=70s | ρ=876kg/m3 | KPX=1.0750 | qmPX=0.168kg/kg |
| DSMB=600cm | T=177℃ | ε=0.39 | KOX=0.2850 | qmOX=0.168kg/kg |
| 床层数分配7-9-5-3 | p=0.88MPa | dp=0.56mm | KMX=0.2645 | qmMX=0.168kg/kg |
| Pe=2000 | KPDEB=1.3125 | qmPDEB=0.138kg/kg | ||
| KL=9min-1 | ||||
| 流股 | 模拟移动床 | 反应精馏 | 结晶 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDEB | S101 | PX | MX | OX | DTBB,TBB | S202 | PX | OX | TBMX | S301 | PX | MX | OX | |||
| 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量/kmol·h-1 | 物流量/kmol·h-1 | 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量/kmol·h-1 | 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量 /kmol·h-1 | 物流量 /kmol·h-1 | |||
| A | 156.64 | 96.44 | 98.38 | 52.19 | 22.38 | 79.89 | 45.34 | 94.94 | 22.38 | 50.60 | 10.67 | 89.33 | 52.25 | 22.41 | ||
| B | 167.80 | 95.36 | 97.42 | 47.04 | 18.62 | 72.00 | 50.08 | 95.32 | 18.70 | 46.08 | 8.68 | 91.32 | 47.14 | 18.66 | ||
| C | 194.43 | 93.88 | 96.87 | 26.31 | 24.65 | 40.28 | 71.15 | 96.77 | 24.75 | 26.04 | 6.22 | 93.78 | 26.39 | 24.72 | ||
| D | 235.55 | 92.43 | 96.27 | 19.70 | 8.88 | 30.15 | 77.90 | 97.43 | 8.93 | 19.70 | 3.14 | 96.86 | 19.80 | 8.92 | ||
| E | 274.96 | 95.69 | 95.16 | 12.01 | 5.83 | 18.39 | 85.44 | 97.94 | 5.89 | 12.14 | 2.57 | 97.43 | 12.09 | 5.87 | ||
| F | 310.32 | 98.26 | 94.22 | 3.90 | 3.85 | 5.97 | 94.46 | 98.38 | 3.92 | 3.94 | 0.09 | 99.91 | 3.93 | 3.88 | ||
| 流股 | 模拟移动床 | 反应精馏 | 结晶 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDEB | S101 | PX | MX | OX | DTBB,TBB | S202 | PX | OX | TBMX | S301 | PX | MX | OX | |||
| 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量/kmol·h-1 | 物流量/kmol·h-1 | 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量/kmol·h-1 | 物流量/kmol·h-1 | PX质量分数/% | PX收率/% | 物流量 /kmol·h-1 | 物流量 /kmol·h-1 | |||
| A | 156.64 | 96.44 | 98.38 | 52.19 | 22.38 | 79.89 | 45.34 | 94.94 | 22.38 | 50.60 | 10.67 | 89.33 | 52.25 | 22.41 | ||
| B | 167.80 | 95.36 | 97.42 | 47.04 | 18.62 | 72.00 | 50.08 | 95.32 | 18.70 | 46.08 | 8.68 | 91.32 | 47.14 | 18.66 | ||
| C | 194.43 | 93.88 | 96.87 | 26.31 | 24.65 | 40.28 | 71.15 | 96.77 | 24.75 | 26.04 | 6.22 | 93.78 | 26.39 | 24.72 | ||
| D | 235.55 | 92.43 | 96.27 | 19.70 | 8.88 | 30.15 | 77.90 | 97.43 | 8.93 | 19.70 | 3.14 | 96.86 | 19.80 | 8.92 | ||
| E | 274.96 | 95.69 | 95.16 | 12.01 | 5.83 | 18.39 | 85.44 | 97.94 | 5.89 | 12.14 | 2.57 | 97.43 | 12.09 | 5.87 | ||
| F | 310.32 | 98.26 | 94.22 | 3.90 | 3.85 | 5.97 | 94.46 | 98.38 | 3.92 | 3.94 | 0.09 | 99.91 | 3.93 | 3.88 | ||
| 参数 | 模拟移动床 | |||||||
|---|---|---|---|---|---|---|---|---|
| 流股 | FEED | PDEB | S101 | S102 | S103 | PX | MX | OX |
| 温度/℃ | 150.00 | 25.00 | 138.00 | 138.00 | 153.00 | 132.00 | 139.00 | 174.00 |
| 压力/MPa | 0.30 | 0.10 | 0.20 | 0.20 | 0.15 | 0.15 | 0.12 | 0.15 |
| 摩尔流量/kmol·h-1 | 100.00 | 235.55 | 254.43 | 81.10 | 29.54 | 66.05 | 20.41 | 9.13 |
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 0 | 69.86 | 0.95 | 0.80 | 65.85 | 0.71 | 0.09 |
| MX | 20.10 | 0 | 0.24 | 19.86 | 19.86 | 0.20 | 19.70 | 0.16 |
| OX | 9.06 | 0 | 0.16 | 8.90 | 8.88 | 0 | 0 | 8.88 |
| PDEB | 0 | 235.55 | 184.17 | 51.39 | 0 | 0 | 0 | 0 |
| 参数 | 反应精馏 | |||||||
| 流股 | FEED | DTBB,TBB | S201 | S202 | B | PX | TBMX | OX |
| 温度/℃ | 150.00 | 150.00 | 132.00 | 97.00 | 120.00 | 184.00 | 188.00 | 178.00 |
| 压力/MPa | 0.30 | 0.20 | 0.25 | 0.20 | 0.18 | 0.20 | 0.25 | 0.30 |
| 摩尔流量/kmol·h-1 | 100.00 | 30.15 | 90.71 | 86.43 | 15.87 | 70.31 | 19.42 | 9.26 |
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 0 | 70.56 | 70.56 | 0 | 70.31 | 0 | 0.25 |
| MX | 20.10 | 0 | 20.02 | 0 | 0 | 0 | 0 | 0.08 |
| OX | 9.06 | 0 | 0.13 | 0 | 0 | 0 | 0 | 8.93 |
| DTBB | 0 | 20.10 | 0 | 0 | 0 | 0 | 0 | 0 |
| TBB | 0 | 10.05 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 0 | 0 | 0 | 15.87 | 15.87 | 0 | 0 | 0 |
| TBMX | 0 | 0 | 0 | 0 | 0 | 0 | 19.42 | 0 |
| 参数 | 深冷结晶 | |||||||
| 流股 | FEED | S301 | S302 | PX | MX | OX | ||
| 温度/℃ | 150.00 | -30.00 | 138.00 | -30.00 | 132.00 | 153.00 | ||
| 压力/MPa | 0.30 | 0.20 | 0.25 | 0.30 | 0.23 | 0.25 | ||
| 摩尔流量/kmol·h-1 | 100.00 | 30.97 | 30.97 | 69.00 | 21.24 | 9.73 | ||
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 1.81 | 1.81 | 69.00 | 1.30 | 0.51 | ||
| MX | 20.10 | 20.10 | 20.10 | 0 | 19.80 | 0.30 | ||
| OX | 9.06 | 9.06 | 9.06 | 0 | 0.14 | 8.92 | ||
| 参数 | 模拟移动床 | |||||||
|---|---|---|---|---|---|---|---|---|
| 流股 | FEED | PDEB | S101 | S102 | S103 | PX | MX | OX |
| 温度/℃ | 150.00 | 25.00 | 138.00 | 138.00 | 153.00 | 132.00 | 139.00 | 174.00 |
| 压力/MPa | 0.30 | 0.10 | 0.20 | 0.20 | 0.15 | 0.15 | 0.12 | 0.15 |
| 摩尔流量/kmol·h-1 | 100.00 | 235.55 | 254.43 | 81.10 | 29.54 | 66.05 | 20.41 | 9.13 |
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 0 | 69.86 | 0.95 | 0.80 | 65.85 | 0.71 | 0.09 |
| MX | 20.10 | 0 | 0.24 | 19.86 | 19.86 | 0.20 | 19.70 | 0.16 |
| OX | 9.06 | 0 | 0.16 | 8.90 | 8.88 | 0 | 0 | 8.88 |
| PDEB | 0 | 235.55 | 184.17 | 51.39 | 0 | 0 | 0 | 0 |
| 参数 | 反应精馏 | |||||||
| 流股 | FEED | DTBB,TBB | S201 | S202 | B | PX | TBMX | OX |
| 温度/℃ | 150.00 | 150.00 | 132.00 | 97.00 | 120.00 | 184.00 | 188.00 | 178.00 |
| 压力/MPa | 0.30 | 0.20 | 0.25 | 0.20 | 0.18 | 0.20 | 0.25 | 0.30 |
| 摩尔流量/kmol·h-1 | 100.00 | 30.15 | 90.71 | 86.43 | 15.87 | 70.31 | 19.42 | 9.26 |
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 0 | 70.56 | 70.56 | 0 | 70.31 | 0 | 0.25 |
| MX | 20.10 | 0 | 20.02 | 0 | 0 | 0 | 0 | 0.08 |
| OX | 9.06 | 0 | 0.13 | 0 | 0 | 0 | 0 | 8.93 |
| DTBB | 0 | 20.10 | 0 | 0 | 0 | 0 | 0 | 0 |
| TBB | 0 | 10.05 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 0 | 0 | 0 | 15.87 | 15.87 | 0 | 0 | 0 |
| TBMX | 0 | 0 | 0 | 0 | 0 | 0 | 19.42 | 0 |
| 参数 | 深冷结晶 | |||||||
| 流股 | FEED | S301 | S302 | PX | MX | OX | ||
| 温度/℃ | 150.00 | -30.00 | 138.00 | -30.00 | 132.00 | 153.00 | ||
| 压力/MPa | 0.30 | 0.20 | 0.25 | 0.30 | 0.23 | 0.25 | ||
| 摩尔流量/kmol·h-1 | 100.00 | 30.97 | 30.97 | 69.00 | 21.24 | 9.73 | ||
| 组分流量/kmol·h-1 | ||||||||
| PX | 70.81 | 1.81 | 1.81 | 69.00 | 1.30 | 0.51 | ||
| MX | 20.10 | 20.10 | 20.10 | 0 | 19.80 | 0.30 | ||
| OX | 9.06 | 9.06 | 9.06 | 0 | 0.14 | 8.92 | ||
| 参数 | 公用工程 | 原料 | 产品 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 急冷水 | 低压蒸汽 | 中压蒸汽 | 工业用电 | 混合二甲苯 | DTBB/TBB | PX,MX,OX | TBMX | B | |
| 价格 | 5CNY/t | 120CNY/t | 180CNY/t | 0.38CNY/kWh | 5900CNY/t | 7700CNY/t | 7600CNY/t | 7700CNY/t | 7600CNY/t |
| 参数 | 公用工程 | 原料 | 产品 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 急冷水 | 低压蒸汽 | 中压蒸汽 | 工业用电 | 混合二甲苯 | DTBB/TBB | PX,MX,OX | TBMX | B | |
| 价格 | 5CNY/t | 120CNY/t | 180CNY/t | 0.38CNY/kWh | 5900CNY/t | 7700CNY/t | 7600CNY/t | 7700CNY/t | 7600CNY/t |
| [4] | MINCEVA Mirjana, GOMES Pedro Sá, MESHKO Vera, et al. Simulated moving bed reactor for isomerization and separation of p-xylene[J]. Chemical Engineering Journal, 2008, 140(1/2/3): 305-323. |
| [5] | GONÇALVES Jonathan C, FARIA Rui P V, FERREIRA Alexandre F P, et al. Optimization of a simulated moving bed unit within an existing and revamped aromatics complex with crystallization and toluene methylation units[J]. Industrial & Engineering Chemistry Research, 2020, 59(25): 11570-11581. |
| [6] | 刘莹, 郑芳, 杨启炜, 等. 二甲苯异构体吸附分离研究进展[J]. 化工学报, 2024, 75(4): 1081-1095. |
| LIU Ying, ZHENG Fang, YANG Qiwei, et al. Recent progress in adsorption and separation of xylene isomers[J]. CIESC Journal, 2024, 75(4): 1081-1095. | |
| [7] | ASHRAF Muhammad Tahir, CHEBBI Rachid, DARWISH Naif A. Process of p-xylene production by highly selective methylation of toluene[J]. Industrial & Engineering Chemistry Research, 2013, 52(38): 13730-13737. |
| [8] | SAITO Shozaburo, MICHISHITA Tsuguo, MAEDA Siro. Separation of meta- and para-xylene mixture by distillation accompanied by chemical reactions[J]. Journal of Chemical Engineering of Japan, 1971, 4(1): 37-43. |
| [9] | 张方坤, 王云龙, 徐啟蕾, 等. 一种萃取精馏分离邻/间/对二甲苯混合物的方法: CN117285405A[P]. 2023-12-26. |
| ZHANG Fangkun, Wang Yunlong, Xu Qilei, et al. A method for separating o/m/p-xylene mixture by extractive distillation: CN117285405A[P] 2023-12-26. | |
| [10] | 赵同复, 李斌, 王振龙, 等. BaX分子筛的阳离子分布及其吸附分离对二甲苯的机理[J]. 催化学报, 2005, 26(8): 655-659. |
| ZHAO Tongfu, LI Bin, WANG Zhenlong, et al. Distribution of cation in BaX zeolite and mechanism of adsorption and separation for paraxylene[J]. Chinese Journal of Catalysis, 2005, 26(8): 655-659. | |
| [11] | RASOULI Milad, YAGHOBI Nakisa, MOVASSAGHI GILANI Seyedeh Zahra, et al. Influence of monovalent alkaline metal cations on binder-free nano-zeolite X in para-xylene separation[J]. Chinese Journal of Chemical Engineering, 2015, 23(1): 64-70. |
| [12] | CHEN Weiye, YAO Tuo, LIU Jian, et al. Modeling and validation of multi-objective optimization for mixed xylene hybrid distillation/crystallization process[J]. Separation and Purification Technology, 2025, 354: 128778. |
| [13] | 杨勇, 张钊, 王东亮, 等. 基于CO2加氢耦合甲苯甲基化选择催化的PX生产工艺对比[J]. 清华大学学报(自然科学版), 2024, 64(3): 538-544. |
| YANG Yong, ZHANG Zhao, WANG Dongliang, et al. Production technology of p-xylene production by toluene methylation with selective carbon dioxide hydrogenation[J]. Journal of Tsinghua University (Science and Technology), 2024, 64(3): 538-544. | |
| [14] | 夏敦焰, 彭莉, 吴政奇, 等. MFI型分子筛膜的两段变温合成及对二甲苯异构体的分离性能[J]. 高等学校化学学报, 2020, 41(12): 2813-2821. |
| XIA Dunyan, PENG Li, WU Zhengqi, et al. Two-stage varying-temperature synthesis of MFI zeolite membrane and their separation performance for xylene isomers[J]. Chemical Journal of Chinese Universities, 2020, 41(12): 2813-2821. | |
| [15] | ZHOU Jian, LIU Zhicheng, WANG Yangdong, et al. Shape selective catalysis in methylation of toluene: Development, challenges and perspectives[J]. Frontiers of Chemical Science and Engineering, 2018, 12(1): 103-112. |
| [16] | SHARMA Poonam, SEBASTIAN Joby, GHOSH Sreetama, et al. Recent advances in hydrogenation of CO2 into hydrocarbons via methanol intermediate over heterogeneous catalysts[J]. Catalysis Science & Technology, 2021, 11(5): 1665-1697. |
| [17] | CHAKINALA Nandana, CHAKINALA Anand G. Process design strategies to produce p-xylene via toluene methylation: A review[J]. Industrial & Engineering Chemistry Research, 2021, 60(15): 5331-5351. |
| [18] | LIU Jing, YANG Yu, WEI Shunan, et al. Intensified p-xylene production process through toluene and methanol alkylation[J]. Industrial & Engineering Chemistry Research, 2018, 57(38): 12829-12841. |
| [19] | WANG Dongliang, ZHANG Junqiang, YANG Yong, et al. Process simulation for enhanced p-xylene production via aromatics complex integrated toluene methylation with low-cost methanol feedstock[J]. Chemical Engineering Research and Design, 2023, 191: 184-195. |
| [20] | SHANG Xin, ZHUO Hongying, HAN Qiao, et al. Xylene synthesis through tandem CO2 hydrogenation and toluene methylation over a composite ZnZrO zeolite catalyst[J]. Angewandte Chemie International Edition, 2023, 62(37): e202309377. |
| [21] | ZUO Jiachang, CHEN Weikun, LIU Jia, et al. Selective methylation of toluene using CO2 and H2 to para-xylene[J]. Science Advances, 2020, 6(34): eaba5433. |
| [22] | MIAO Dengyun, PAN Xiulian, JIAO Feng, et al. Selective synthesis of para-xylene and light olefins from CO2/H2 in the presence of toluene[J]. Catalysis Science & Technology, 2021, 11(13): 4521-4528. |
| [1] | BUSCA Guido. Production of gasolines and monocyclic aromatic hydrocarbons: From fossil raw materials to green processes[J]. Energies, 2021, 14(13): 4061. |
| [2] | PENG Bo, WANG Shaoyan. Separation of p-xylene and m-xylene by simulated moving bed chromatography with MIL-53(Fe) as stationary phase[J]. Journal of Chromatography A, 2022, 1673: 463091. |
| [3] | GONÇALVES Jonathan C, RODRIGUES Alírio E. Simulated moving bed reactor for p-xylene production: Adsorbent and catalyst homogeneous mixture[J]. Chemical Engineering Journal, 2014, 258: 194-202. |
| [23] | SHI Qian, GONÇALVES Jonathan C, FERREIRA Alexandre F P, et al. A review of advances in production and separation of xylene isomers[J]. Chemical Engineering and Processing: Process Intensification, 2021, 169: 108603. |
| [24] | ZHANG Peipei, TAN Li, YANG Guohui, et al. One-pass selective conversion of syngas to para-xylene[J]. Chemical Science, 2017, 8(12): 7941-7946. |
| [25] | HAN Xiaoqin, ZUO Jiachang, WEN Danlu, et al. Toluene methylation with syngas to para-xylene by bifunctional ZnZrO x -HZSM-5 catalysts[J]. Chinese Journal of Catalysis, 2022, 43(4): 1156-1164. |
| [26] | TIAN Haifeng, HE Huanhuan, JIAO Jiapeng, et al. Tandem catalysts composed of different morphology HZSM-5 and metal oxides for CO2 hydrogenation to aromatics[J]. Fuel, 2022, 314: 123119. |
| [27] | LI Wen, WANG Kuncan, HUANG Junjie, et al. M x O y -ZrO2 (M=Zn, Co, Cu) solid solutions derived from schiff base-bridged UiO-66 composites as high-performance catalysts for CO2 hydrogenation[J]. ACS Applied Materials & Interfaces, 2019, 11(36): 33263-33272. |
| [28] | XIAO Zhikai, HUANG Hai, CAO Chenxi, et al. Designing a bifunctional ZrCuO x /HZSM-5 catalyst for selective methylation of toluene with carbon dioxide to para-xylene[J]. Fuel, 2022, 319: 123848. |
| [29] | NI Youming, CHEN Zhiyang, FU Yi, et al. Selective conversion of CO2 and H2 into aromatics[J]. Nature Communications, 2018, 9(1): 3457. |
| [30] | Mahdi ABDI-KHANGHAH, ALRASHED Abdullah A A A, HAMOULE Touba,et al. Toluene methylation to para-xylene[J]. Journal of Thermal Analysis and Calorimetry, 2018, 135(3): 1723-1732. |
| [31] | 杨明磊, 魏民, 胡蓉, 等. 二甲苯模拟移动床分离过程建模与仿真[J]. 化工学报, 2013, 64(12): 4335-4341. |
| YANG Minglei, WEI Min, HU Rong, et al. Modeling of simulated moving bed for xylene separation[J]. CIESC Journal, 2013, 64(12): 4335-4341. | |
| [32] | 张东辉, 沈圆辉, 吴丽梅, 等. 对二甲苯模拟移动床分离过程的模拟与优化[J]. 天津大学学报(自然科学与工程技术版), 2016, 49(3): 279-286. |
| ZHANG Donghui, SHEN Yuanhui, WU Limei, et al. Simulation and optimization of simulated moving bed for the separation of p-xylene[J]. Journal of Tianjin University (Science and Technology), 2016, 49(3): 279-286. | |
| [33] | WANKAT P C. Simulated moving bed cascades for ternary separations[J]. Industrial & Engineering Chemistry Research, 2001, 40(26): 6185-6193. |
| [34] | JIN Weihua, WANKAT Phillip C. Hybrid simulated moving bed processes for the purification of p‐xylene[J]. Separation Science and Technology, 2007, 42(4): 669-700. |
| [35] | Cristofer BRAVO-BRAVO, SEGOVIA-HERNÁNDEZ Juan Gabriel, Salvador HERNÁNDEZ, et al. Hybrid distillation/melt crystallization process using thermally coupled arrangements: Optimization with evolutive algorithms[J]. Chemical Engineering and Processing: Process Intensification, 2013, 67: 25-38. |
| [36] | KONG Lingxun, WU Yaqing, MARAVELIAS Christos T. Simultaneous utility and heat exchanger area targeting for integrated process synthesis and heat integration[J]. Industrial & Engineering Chemistry Research, 2017, 56(41): 11847-11859. |
| [37] | IBRAHIM Dauda, JOBSON Megan, Gonzalo GUILLÉN-GOSÁLBEZ. Optimization-based design of crude oil distillation units using rigorous simulation models[J]. Industrial & Engineering Chemistry Research, 2017, 56(23): 6728-6740. |
| [1] | ZONG Huajian, LI Ying, ZHANG Xiangping. Energy integration and carbon flow analysis of process of CO2 chemical transformation to dimethyl carbonate and ethylene glycol [J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5369-5380. |
| [2] | CHEN Le, CHONG Hailing, ZHANG Zhihui, HE Mingyang, CHEN Qun. Synthesis of Cu-BTC modified by CTAB and its adsorption and separation of xylene isomers [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 455-464. |
| [3] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [4] | HAN Wentao, HAN Zhenwei, LI Hong, GAO Xin, LI Xingang. Simulation of reactive distillation for the synthesis of ethyl levulinate and energy saving optimization of dividing wall column [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1759-1769. |
| [5] | LAI Jianing, GAO Xin, CONG Haifeng, LI Hong, LI Xingang. Simulation and optimization of synthesizing solketal by reactive distillation process [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3584-3590. |
| [6] | WANG Xiaoda, CHEN Yu, WANG Qinglian, HUANG Zhixian, YANG Chen, WANG Hongxing, QIU Ting. Review on etherification by reactive distillation [J]. Chemical Industry and Engineering Progress, 2021, 40(4): 1797-1811. |
| [7] | WANG Honghai, YIN Yi, LIU Xing, WEI Siwen, SU Weiyi, LI Chunli. Preparation and optimization of enzyme-loaded plastic packing [J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6829-6838. |
| [8] | Zhengxi YU, Shuliang XU, Tao ZHANG, Mao YE, Zhongmin LIU. Research progress and development trend in para-xylene production technology [J]. Chemical Industry and Engineering Progress, 2020, 39(12): 4984-4992. |
| [9] | Xueli GENG, Ying MENG, Haifeng CONG, Hong LI, Xin GAO, Xingang LI. Review on the synthesis process and industrialization of polyoxymethylene dimethyl ethers [J]. Chemical Industry and Engineering Progress, 2020, 39(12): 4993-5008. |
| [10] | Jiaqi LIU, Lianyong LIU, Shuangyu WANG, Zhihui LI, Yanhua ZHANG, Xiaoshu DING, Dongsheng ZHANG, Xinqiang ZHAO, Yanji WANG. Research progress of ketoxime hydrolysis reaction and its hydroxylamine product separation [J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4147-4154. |
| [11] | Jiansong HUANG,Songlin XU. Optimization and comparison of different distillation schemes for anhydrous tert-butanol production [J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5181-5188. |
| [12] | DONG Yanbo, HE Ruining, MU Chunxia, ZOU Yun, TONG Zhangfa. Process simulation of synthesis of ethyl acetate by reactive distillation catalyzed by ionic liquid [J]. Chemical Industry and Engineering Progress, 2018, 37(02): 468-474. |
| [13] | LING Xiaomei, ZHENG Weiyue, WANG Xiaoda, QIU Ting. Advances in technology of reactive dividing wall column [J]. Chemical Industry and Engineering Progress, 2017, 36(08): 2776-2786. |
| [14] | LI Hong, MENG Ying, LI Xin'gang, GAO Xin . Dynamic simulation and analysis of reactive distillation column for production of amyl acetate [J]. Chemical Industry and Engineering Progree, 2015, 34(12): 4165-4171. |
| [15] | ZHANG Huanhuan, GE Zhiqiang, GUO Xianghai, BAI Peng. A review on recovery technologies of acetic acid from industrial wastewater [J]. Chemical Industry and Engineering Progree, 2015, 34(06): 1768-1778. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||