Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (1): 273-281.DOI: 10.16085/j.issn.1000-6613.2020-0501
• Materials science and technology • Previous Articles Next Articles
Research progress in the application of heteroatom-doped carbonaceous materials for persulfate activation
Xiaojuan LI1( ), Lanmei YE1, Fengzhen LIAO1, Ziyu YE1, Lizhi YEH1,2
), Lanmei YE1, Fengzhen LIAO1, Ziyu YE1, Lizhi YEH1,2
- 1.College of Environment and Resources, Fuzhou University, Fuzhou 350108, Fujian, China
2.Department of Civil and Environmental Engineering, University of Kaohsiung, Kaohsiung 81148, Taiwan, China
-
Received:2020-04-01Online:2021-01-12Published:2021-01-05 -
Contact:Xiaojuan LI
杂原子掺杂碳材料活化过硫酸盐技术的研究进展
李小娟1( ), 叶兰妹1, 廖凤珍1, 叶梓瑜1, 叶礼志1,2
), 叶兰妹1, 廖凤珍1, 叶梓瑜1, 叶礼志1,2
- 1.福州大学环境与资源学院,福建 福州 350108
2.高雄大学土木与环境工程学系,台湾 高雄 81148
-
通讯作者:李小娟 -
作者简介:李小娟(1982—),女,博士,副教授,研究方向为高效环境材料的制备与应用。E-mail:lixiaojuan@fzu.edu.cn 。 -
基金资助:国家自然科学基金(21407026)
CLC Number:
Cite this article
Xiaojuan LI, Lanmei YE, Fengzhen LIAO, Ziyu YE, Lizhi YEH. Research progress in the application of heteroatom-doped carbonaceous materials for persulfate activation[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 273-281.
李小娟, 叶兰妹, 廖凤珍, 叶梓瑜, 叶礼志. 杂原子掺杂碳材料活化过硫酸盐技术的研究进展[J]. 化工进展, 2021, 40(1): 273-281.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-0501
| 单一杂原子 掺杂碳材料 | 掺杂方法 | 碳基体 /前体 | 掺杂 元素 | 杂原子源 | 活化过硫酸盐降解有机污染物效果 | 机理① | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
催化剂浓度 /mg·L-1 | 过硫酸盐投加量 | 污染物浓度 | 降解效果 | |||||||
| N-SWCNT | 液相 | SWCNT | N | 尿素 | 200 | 2.0g·L-1 PDS | 20mg·kg-1 硝基苯 | 60min,100% | R | [ |
| NMC-850 | 液相 | SBA-15 | N | 乙二胺和 双氰胺 | 50 | 1.25g·L-1 PMS | 30mg·L-1 邻苯基苯酚 | 150min,81% | 1O2 | [ |
| NCNT-550 | 液相 | CNT | N | 尿素 | 100 | 8mmol·L-1 PMS | 0.106mmol·L-1 苯酚 | 20min,100% | 1O2 | [ |
| N-ND/PDDA/GO | 液相 | ND/PDDA/GO | N | NH3 | 100 | 1mmol·L-1 PMS | 0.1mmol·L-1 4-CP | 60min,100% | E | [ |
| N-RGO | 液相 | rGO | N | 氨溶液 | 120 | 0.8mmol·L-1 PMS | 88mg·L-1 BPA | 7min,100% | R | [ |
| N-rGO | 液相 | rGO | N | 三聚氰胺 | 50 | 2g·L-1 PMS | 20mg·L-1 IBP | 180min,90% | R | [ |
| N-rGO-N2 | 液相 | rGO | N | 尿素 | 400 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 20min,100% | R+1O2 | [ |
| N-AND | 液相 | AND | N | 三聚氰胺 | 200 | 6.5mmol·L-1 PMS | 20mg·L-1 苯酚 | 45min,100% | R | [ |
| N-CNT-35 | 液相 | CNT | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 45min,100% | R | [ |
| NoCNT-700 | 液相 | SWCNTs | N | 三聚氰胺 | 100 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 20min,100% | R+E | [ |
| NG350 | 液相 | rGO | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 180min,85% | R | [ |
| G-N | 液相 | GO | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 45min,100% | — | [ |
| NG-700 | 液相 | rGO | N | 三聚氰胺 | 100 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 30min,100% | R | [ |
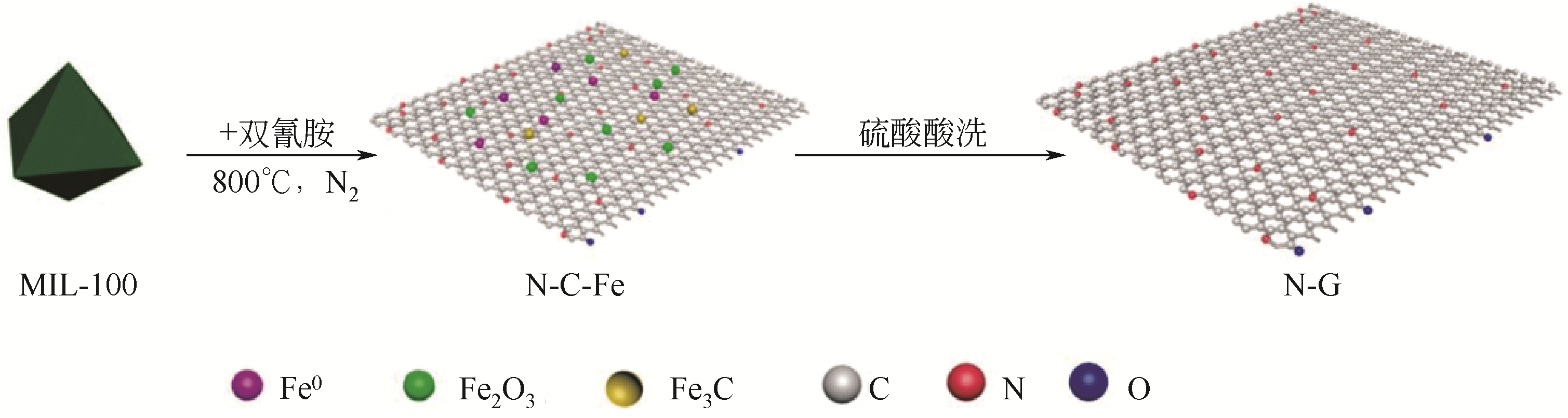
N-G(D)N-G(M) N-G(M) | 液相 | MIL-100 | N | 双氰胺 三聚氰胺 尿素 | 100 | 3.25mmol·L-1 PMS | 20mg·kg-1 PHBA | 20min,100% 30min,100% 90min,100% | 1O2 | [ |
| N-G(D) | 液相 | MIL-100 | N | 双氰胺 | 100 | 3.25mmol·L-1 PMS | 50mg·kg-1 苯酚 | 30min,100% | 1O2 | [ |
| NH4NO3-CNT-OH | 固相 | CNT-OH (0.5∶1) | N | 硝酸 | 100 | 污染物∶[PDS]= 1∶500 | 43.48mmol·L-1 2,4,4-HBP | 120min,100% | R | [ |
| N-IrGO | 固相 | IrGO | N | 尿素 | 50 | 500mg·L-1 PMS | 5mg·L-1 二苯甲酮-1 | 60min,100% | 1O2 | [ |
| CPPy-F-8 | 原位 | PPy-F | N | 聚吡咯 | 100 | 3.25mmol·L-1 PMS | 20mg·L-1 苯酚 | 120min,97% | R+1O2 | [ |
| NPC-800 | 原位 | ZIF-8 | N | 2-甲基咪唑 | 200 | 0.8g·L-1 PMS | 25mg·L-1 苯酚 | 60min,86.1% | R | [ |
| N-C-900 | 原位 | ZIF-8 | N | 2-甲基咪唑 | 150 | 5mmol·L-1 PDS | 0.3mmol·L-1 PCA | 120min, >96.3% | R+1O2+E | [ |
| ACS-800 | 原位 | 聚噻吩 | S | 噻吩 | 50 | 8mmol·L-1 PDS | 40mg·L-1 4-CP | 60min,100% | R | [ |
| SDAC-800 | 原位 | 聚噻吩 | S | 噻吩 | 100 | 15mmol·L-1 PDS | 80mg·L-1 4-CP | 90min,100% | R+E | [ |
| B-OMC | 液相 | 酚醛树脂 | B | 硼酸 | 200 | 1?mmol·L-1 PMS | 20mg·L-1 BPA | 60min,91% | 1O2 | [ |
| NPCs | 原位 | ZIF-8 | N | 2-甲基咪唑 | 200 | 1.6mmol·L-1 PMS | 20mg·kg-1 苯酚 | 60min,100% | R | [ |
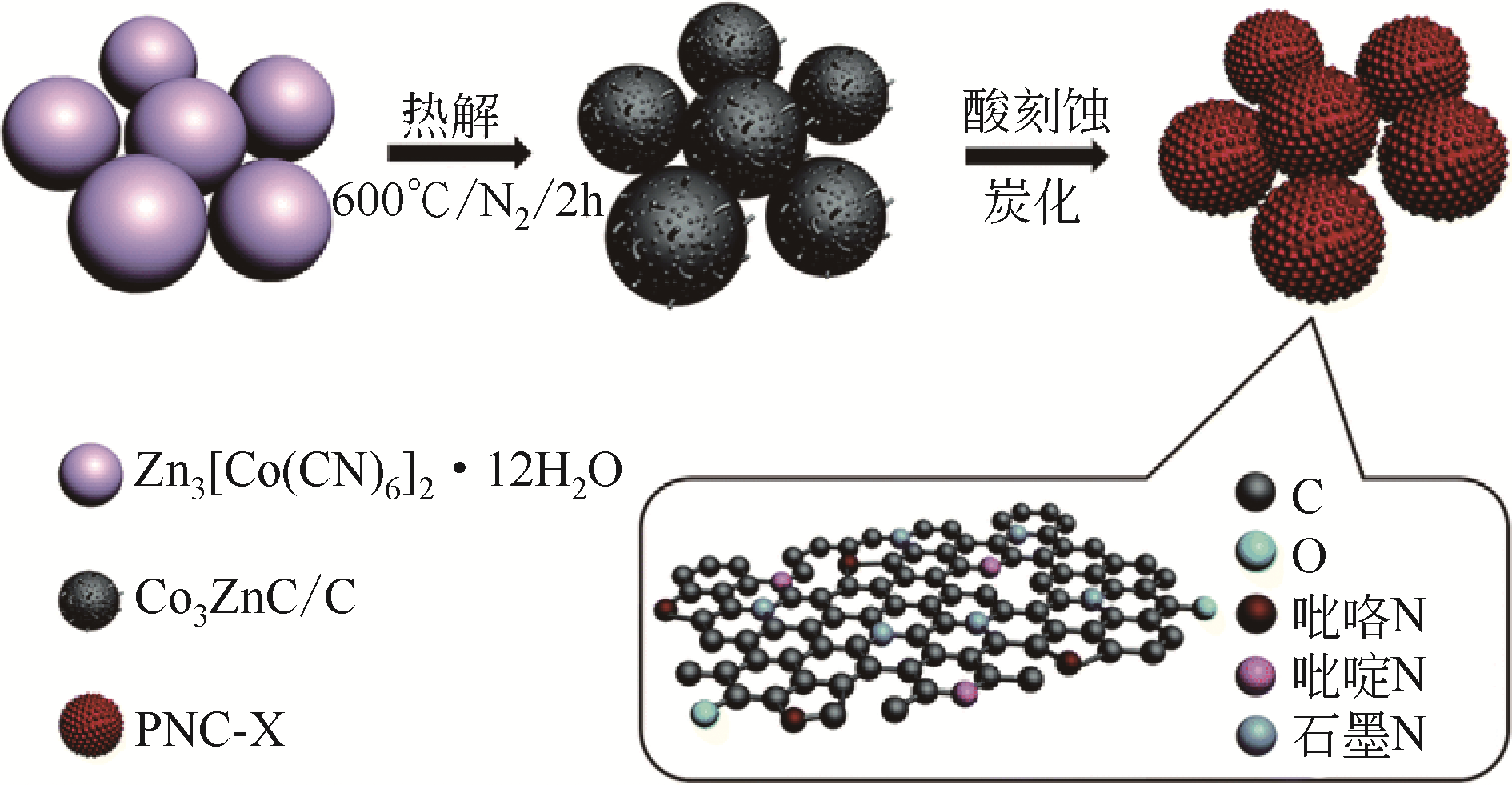
| PNC-800 | 原位 | Zn-Co PBAs | N | 钴氰化钾 | 100 | 1.0g·L-1 PMS | 100mg·L-1 MB | 10min,100% | R+1O2 | [ |
| CBs@NCCs | 原位 | Co-Fe PBAs | N | 钴氰化钾 | 60 | 1.0g·L-1 PMS | 100mg·L-1 MB | 60min, >95% | R+1O2 | [ |
| 单一杂原子 掺杂碳材料 | 掺杂方法 | 碳基体 /前体 | 掺杂 元素 | 杂原子源 | 活化过硫酸盐降解有机污染物效果 | 机理① | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
催化剂浓度 /mg·L-1 | 过硫酸盐投加量 | 污染物浓度 | 降解效果 | |||||||
| N-SWCNT | 液相 | SWCNT | N | 尿素 | 200 | 2.0g·L-1 PDS | 20mg·kg-1 硝基苯 | 60min,100% | R | [ |
| NMC-850 | 液相 | SBA-15 | N | 乙二胺和 双氰胺 | 50 | 1.25g·L-1 PMS | 30mg·L-1 邻苯基苯酚 | 150min,81% | 1O2 | [ |
| NCNT-550 | 液相 | CNT | N | 尿素 | 100 | 8mmol·L-1 PMS | 0.106mmol·L-1 苯酚 | 20min,100% | 1O2 | [ |
| N-ND/PDDA/GO | 液相 | ND/PDDA/GO | N | NH3 | 100 | 1mmol·L-1 PMS | 0.1mmol·L-1 4-CP | 60min,100% | E | [ |
| N-RGO | 液相 | rGO | N | 氨溶液 | 120 | 0.8mmol·L-1 PMS | 88mg·L-1 BPA | 7min,100% | R | [ |
| N-rGO | 液相 | rGO | N | 三聚氰胺 | 50 | 2g·L-1 PMS | 20mg·L-1 IBP | 180min,90% | R | [ |
| N-rGO-N2 | 液相 | rGO | N | 尿素 | 400 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 20min,100% | R+1O2 | [ |
| N-AND | 液相 | AND | N | 三聚氰胺 | 200 | 6.5mmol·L-1 PMS | 20mg·L-1 苯酚 | 45min,100% | R | [ |
| N-CNT-35 | 液相 | CNT | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 45min,100% | R | [ |
| NoCNT-700 | 液相 | SWCNTs | N | 三聚氰胺 | 100 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 20min,100% | R+E | [ |
| NG350 | 液相 | rGO | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 180min,85% | R | [ |
| G-N | 液相 | GO | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 45min,100% | — | [ |
| NG-700 | 液相 | rGO | N | 三聚氰胺 | 100 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 30min,100% | R | [ |
N-G(D)N-G(M) N-G(M) | 液相 | MIL-100 | N | 双氰胺 三聚氰胺 尿素 | 100 | 3.25mmol·L-1 PMS | 20mg·kg-1 PHBA | 20min,100% 30min,100% 90min,100% | 1O2 | [ |
| N-G(D) | 液相 | MIL-100 | N | 双氰胺 | 100 | 3.25mmol·L-1 PMS | 50mg·kg-1 苯酚 | 30min,100% | 1O2 | [ |
| NH4NO3-CNT-OH | 固相 | CNT-OH (0.5∶1) | N | 硝酸 | 100 | 污染物∶[PDS]= 1∶500 | 43.48mmol·L-1 2,4,4-HBP | 120min,100% | R | [ |
| N-IrGO | 固相 | IrGO | N | 尿素 | 50 | 500mg·L-1 PMS | 5mg·L-1 二苯甲酮-1 | 60min,100% | 1O2 | [ |
| CPPy-F-8 | 原位 | PPy-F | N | 聚吡咯 | 100 | 3.25mmol·L-1 PMS | 20mg·L-1 苯酚 | 120min,97% | R+1O2 | [ |
| NPC-800 | 原位 | ZIF-8 | N | 2-甲基咪唑 | 200 | 0.8g·L-1 PMS | 25mg·L-1 苯酚 | 60min,86.1% | R | [ |
| N-C-900 | 原位 | ZIF-8 | N | 2-甲基咪唑 | 150 | 5mmol·L-1 PDS | 0.3mmol·L-1 PCA | 120min, >96.3% | R+1O2+E | [ |
| ACS-800 | 原位 | 聚噻吩 | S | 噻吩 | 50 | 8mmol·L-1 PDS | 40mg·L-1 4-CP | 60min,100% | R | [ |
| SDAC-800 | 原位 | 聚噻吩 | S | 噻吩 | 100 | 15mmol·L-1 PDS | 80mg·L-1 4-CP | 90min,100% | R+E | [ |
| B-OMC | 液相 | 酚醛树脂 | B | 硼酸 | 200 | 1?mmol·L-1 PMS | 20mg·L-1 BPA | 60min,91% | 1O2 | [ |
| NPCs | 原位 | ZIF-8 | N | 2-甲基咪唑 | 200 | 1.6mmol·L-1 PMS | 20mg·kg-1 苯酚 | 60min,100% | R | [ |
| PNC-800 | 原位 | Zn-Co PBAs | N | 钴氰化钾 | 100 | 1.0g·L-1 PMS | 100mg·L-1 MB | 10min,100% | R+1O2 | [ |
| CBs@NCCs | 原位 | Co-Fe PBAs | N | 钴氰化钾 | 60 | 1.0g·L-1 PMS | 100mg·L-1 MB | 60min, >95% | R+1O2 | [ |
| 杂原子 共掺杂碳材料 | 掺杂方法 | 碳基体 /前体 | 掺杂 元素 | 杂原子源 | 活化过硫酸盐降解有机污染物效果 | 机理① | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
催化剂浓度 /mg·L-1 | 过硫酸盐 投加量 | 污染物浓度 | 降解效果 | |||||||
| SNG | 液相 | rGO | N,S | N:硝酸铵 S:二甲基亚砜 | 200 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 90min,100% | R | [ |
| 2sNBG-800 | 液相 | rGO | N,B | N:尿素 B:硼酸 | 200 | 0.5mmol·L-1 PMS | 10mg·L-1 SAM | 20min,100% | R+E | [ |
| N-S-PCs | 液相 | 葡萄糖 | N,S | NS:硫脲 | 50 | 6.5mmol·L-1 PDS | 20mg·L-1 磺胺氯哒嗪 | 20min,100% | R+E | [ |
| NPSC-700 | 液相 | ZIF-8 | N,P,S | NPS:聚丙腈 | 60 | 0.4g·L-1 PMS | 25mg·kg-1 BPA | 30min,90.10% | R | [ |
| SNCs | 液相 | 咖啡粉 | N,S | NS:L-半胱氨酸 | 400 | 2mmol·L-1 PDS | 0.02mmol·L-1 TeC | 60min,100% | 1O2+E | [ |
| i-RGO-NS | 固相 | rGO | N,S | NS:硫脲 | 20 | 307mg·L-1 PMS | 15mg·L-1 MP | 20min,100% | 1O2 | [ |
| N,S-rGO | 固相 | rGO | N,S | NS:硫脲 | 50 | 0.9mmol·L-1 PDS | 2mg·L-1 BPA | 20min,100% | R | [ |
| NS-CNT-COOH | 固相 | CNT | N,S | NS:硫脲 | 100 | 1.0g·L-1 PMS | 0.01g·L-1 BP-4 | 30min,100% | E | [ |
| 杂原子 共掺杂碳材料 | 掺杂方法 | 碳基体 /前体 | 掺杂 元素 | 杂原子源 | 活化过硫酸盐降解有机污染物效果 | 机理① | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
催化剂浓度 /mg·L-1 | 过硫酸盐 投加量 | 污染物浓度 | 降解效果 | |||||||
| SNG | 液相 | rGO | N,S | N:硝酸铵 S:二甲基亚砜 | 200 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 90min,100% | R | [ |
| 2sNBG-800 | 液相 | rGO | N,B | N:尿素 B:硼酸 | 200 | 0.5mmol·L-1 PMS | 10mg·L-1 SAM | 20min,100% | R+E | [ |
| N-S-PCs | 液相 | 葡萄糖 | N,S | NS:硫脲 | 50 | 6.5mmol·L-1 PDS | 20mg·L-1 磺胺氯哒嗪 | 20min,100% | R+E | [ |
| NPSC-700 | 液相 | ZIF-8 | N,P,S | NPS:聚丙腈 | 60 | 0.4g·L-1 PMS | 25mg·kg-1 BPA | 30min,90.10% | R | [ |
| SNCs | 液相 | 咖啡粉 | N,S | NS:L-半胱氨酸 | 400 | 2mmol·L-1 PDS | 0.02mmol·L-1 TeC | 60min,100% | 1O2+E | [ |
| i-RGO-NS | 固相 | rGO | N,S | NS:硫脲 | 20 | 307mg·L-1 PMS | 15mg·L-1 MP | 20min,100% | 1O2 | [ |
| N,S-rGO | 固相 | rGO | N,S | NS:硫脲 | 50 | 0.9mmol·L-1 PDS | 2mg·L-1 BPA | 20min,100% | R | [ |
| NS-CNT-COOH | 固相 | CNT | N,S | NS:硫脲 | 100 | 1.0g·L-1 PMS | 0.01g·L-1 BP-4 | 30min,100% | E | [ |
| 38 | DUAN X G, SUN H Q, WANG Y X, et al. N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation[J]. ACS Catalysis, 2015, 5(2): 553-559. |
| 39 | MA W J, DU Y C, WANG N, et al. ZIF-8 derived nitrogen-doped porous carbon as metal-free catalyst of peroxymonosulfate activation[J]. Environmental Science and Pollution Research, 2017, 24(19): 16276-16288. |
| 40 | LIU Y, MIAO W, FANG X, et al. MOF-derived metal-free N-doped porous carbon mediated peroxydisulfate activation via radical and non-radical pathways: role of graphitic N and C-O[J]. Chemical Engineering Journal, 2020, 380: 122584. |
| 41 | GUO Y P, ZENG Z Q, LI Y L, et al. In-situ sulfur-doped carbon as a metal-free catalyst for persulfate activated oxidation of aqueous organics[J]. Catalysis Today, 2018, 307: 12-19. |
| 42 | GUO Y P, ZENG Z Q, ZHU Y C, et al. Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene[J]. Applied Catalysis B: Environmental, 2018, 220: 635-644. |
| 43 | WANG Y B, LIU M, ZHAO X, et al. Insights into heterogeneous catalysis of peroxymonosulfate activation by boron-doped ordered mesoporous carbon[J]. Carbon, 2018, 135: 238-247. |
| 44 | DUAN X G, INDRAWIRAWAN S, SUN H Q, et al. Effects of nitrogen-, boron-, and phosphorus-doping or codoping on metal-free graphene catalysis[J]. Catalysis Today, 2015, 249: 184-191. |
| 45 | YIN R L, GUO W Q, DU J S, et al. Heteroatoms doped graphene for catalytic ozonation of sulfamethoxazole by metal-free catalysis: performances and mechanisms[J]. Chemical Engineering Journal, 2017, 317: 632-639. |
| 46 | WANG Q, LI L, LUO L, et al. Activation of persulfate with dual-doped reduced graphene oxide for degradation of alkylphenols[J]. Chemical Engineering Journal, 2019, 376: 120891. |
| 47 | TIAN W J, ZHANG H Y, DUAN X G, et al. Nitrogen-and sulfur-codoped hierarchically porous carbon for adsorptive and oxidative removal of pharmaceutical contaminants[J]. ACS Applied Materials and Interfaces, 2016, 8(11): 7184-7193. |
| 48 | SUN H Q, WANG Y X, LIU S Z, et al. Facile synthesis of nitrogen doped reduced graphene oxide as a superior metal-free catalyst for oxidation[J]. Chemical Communications, 2013, 49(85): 9914-9916. |
| 49 | MA W J, WANG N, TONG T Z, et al. Nitrogen, phosphorus, and sulfur tri-doped hollow carbon shells derived from ZIF-67@poly (cyclotriphosphazene-co-4,4′-sulfonyldiphenol) as a robust catalyst of peroxymonosulfate activation for degradation of bisphenol A[J]. Carbon, 2018, 137: 291-303. |
| 1 | WANG J L, WANG S Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. |
| 2 | CHEN X, OH W D, LIM T T. Graphene- and CNTs-based carbocatalysts in persulfates activation: material design and catalytic mechanisms[J]. Chemical Engineering Journal, 2018, 354: 941-976. |
| 3 | GHANBARI F, MORADI M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review[J]. Chemical Engineering Journal, 2017, 310: 41-62. |
| 50 | WANG G L, CHEN S, QUAN X, et al. Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants[J]. Carbon, 2017, 115: 730-739. |
| 51 | DUAN X G, AO Z M, SUN H Q, et al. Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis[J]. ACS Applied Materials and Interfaces, 2015, 7(7): 4169-4178. |
| 52 | LIANG P, ZHANG C, DUAN X G, et al. N-doped graphene from metal-organic frameworks for catalytic oxidation of p-hydroxylbenzoic acid: N-functionality and mechanism[J]. ACS Sustainable Chemistry and Engineering, 2017, 5(3): 2693-2701. |
| 53 | LIANG P, ZHANG C, DUAN X G, et al. An insight into metal organic framework derived N-doped graphene for the oxidative degradation of persistent contaminants: formation mechanism and generation of singlet oxygen from peroxymonosulfate[J]. Environmental Science: Nano, 2017, 4(2): 315-324. |
| 54 | LIU H, SUN P, FENG M B, et al. Nitrogen and sulfur co-doped CNT-COOH as an efficient metal-free catalyst for the degradation of UV filter BP-4 based on sulfate radicals[J]. Applied Catalysis B: Environmental, 2016, 187: 1-10. |
| 55 | YANG L, ZENG X F, WANG W C, et al. Recent progress in MOF-derived, heteroatom-doped porous carbons as highly efficient electrocatalysts for oxygen reduction reaction in fuel cells[J]. Advanced Functional Materials, 2018, 28(7): 1704537. |
| 56 | REN Q, WANG H, LU X F, et al. Recent progress on MOF-derived heteroatom-doped carbon-based electrocatalysts for oxygen reduction reaction[J]. Advanced Science, 2018, 5(3): 1700515. |
| 57 | WANG N, MA W J, REN Z Q, et al. Prussian blue analogues derived porous nitrogen-doped carbon microspheres as high-performance metal-free peroxymonosulfate activators for non-radical-dominated degradation of organic pollutants[J]. Journal of Materials Chemistry A, 2018, 6(3): 884-895. |
| 58 | WANG N, MA W J, REN Z Q, et al. Template synthesis of nitrogen-doped carbon nanocages-encapsulated carbon nanobubbles as catalyst for activation of peroxymonosulfate[J]. Inorganic Chemistry Frontiers, 2018, 5(8): 1849-1860. |
| 59 | HUO X W, ZHOU P, ZHANG J, et al. N, S-doped porous carbons for persulfate activation to remove tetracycline: nonradical mechanism[J]. Journal of Hazardous Materials, 2020, 391: 122055. |
| 60 | SUN P, LIU H, ZHAI Z C, et al. Degradation of UV filter BP-1 with nitrogen-doped industrial graphene as a metal-free catalyst of peroxymonosulfate activation[J]. Chemical Engineering Journal, 2019, 356: 262-271. |
| 4 | DUAN X G, INDRAWIRAWAN S, KANG J, et al. Synergy of carbocatalytic and heat activation of persulfate for evolution of reactive radicals toward metal-free oxidation[J], Catalysis Today, 2020, 355: 319-324. |
| 5 | HUANG K C, ZHAO Z Q, HOAG G E, et al. Degradation of volatile organic compounds with thermally activated persulfate oxidation[J]. Chemosphere, 2005, 61(4): 551-560. |
| 6 | AO X W, LIU W J. Degradation of sulfamethoxazole by medium pressure UV and oxidants: peroxymonosulfate, persulfate, and hydrogen peroxide[J]. Chemical Engineering Journal, 2017, 313: 629-637. |
| 7 | XU L J, WANG X T, SUN Y, et al. Mechanistic study on the combination of ultrasound and peroxymonosulfate for the decomposition of endocrine disrupting compounds[J]. Ultrasonics Sonochemistry, 2020, 60: 104749. |
| 8 | CHUA C K, PUMERA M. Carbocatalysis: the state of “metal-free” catalysis[J]. Chemistry, 2015, 21(36): 12550-12562. |
| 9 | HOU J F, YANG S S, WAN H Q, et al. Highly effective catalytic peroxymonosulfate activation on N-doped mesoporous carbon for o-phenylphenol degradation[J]. Chemosphere, 2018, 197: 485-493. |
| 10 | MANDAL S, BERA T, DUBEY G, et al. Uses of K2S2O8 in metal catalyzed and metal free oxidative transformations[J]. ACS Catalysis, 2018, 8(6): 5085-5144. |
| 11 | DUAN X G, SUN H Q, KANG J, et al. Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons[J]. ACS Catalysis, 2015, 5(8): 4629-4636. |
| 12 | 陈一萍, 夏管商, 郑朝洪, 等. CNTs/PMS高级氧化体系去除水中的环丙沙星[J]. 化工进展, 2019, 38(4): 2037-2045. |
| CHEN Y P, XIA G S, ZHENG C H, et al. Degradation of ciprofloxacin by advanced oxidation process with carbon nanotubes/peroxymonosulfate CNTs/PMS[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 2037-2045. | |
| 13 | YUN E T, YOO H Y, BAE H, et al. Exploring the role of persulfate in the activation process: radical precursor versus electron acceptor[J]. Environmental Science and Technology, 2017, 51(17): 10090-10099. |
| 14 | CHENG X, GUO H G, ZHANG Y L, et al. Insights into the mechanism of nonradical reactions of persulfate activated by carbon nanotubes: activation performance and structure-function relationship[J]. Water Research, 2019, 157: 406-414. |
| 15 | ZHAO Q, MAO Q, ZHOU Y, et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: a review on heterogeneous catalysts and applications[J]. Chemosphere, 2017, 189: 224-238. |
| 16 | OH W D, DONG Z L, Q, LIM T T, et al. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: current development, challenges and prospects[J]. Applied Catalysis B: Environmental, 2016, 194: 169-201. |
| 17 | LI Y, LIU L D, LIU L, et al. Efficient oxidation of phenol by persulfate using manganite as a catalyst[J]. Journal of Molecular Catalysis A: Chemical, 2016, 411, 264-271. |
| 18 | SUN B J, MA W J, WANG N, et al. Polyaniline: a new metal-free catalyst for peroxymonosulfate activation with highly efficient and durable removal of organic pollutants[J]. Environmental Science and Technology, 2019, 53: 9771-9780. |
| 19 | RESHETNYAK O V, KOVAL’CHUK E P, SKURSKI P, et al. The origin of luminescence accompanying electrochemical reduction or chemical decomposition of peroxydisulfates[J]. Journal of Luminescence, 2003, 105(1): 27-34. |
| 20 | HUANG B C, JIANG J, HUANG G X, et al. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate[J]. Journal of Materials Chemistry A, 2018, 6(19): 8978-8985. |
| 21 | WEI M Y, GAO L, LI J, et al. Activation of peroxymonosulfate by graphitic carbon nitride loaded on activated carbon for organic pollutants degradation[J]. Journal of Hazardous Materials, 2016, 316: 60-68. |
| 22 | SUN P, LIU H, FENG M B, et al. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: singlet oxygen-dominated catalytic degradation of organic contaminants[J]. Applied Catalysis B: Environmental, 2019, 251: 335-345. |
| 23 | CHENG X, GUO H G, ZHANG Y L, et al. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes[J]. Water Research, 2017, 113: 80-88. |
| 24 | HOU J F, XU L X, HAN Y X, et al. Deactivation and regeneration of carbon nanotubes and nitrogen-doped carbon nanotubes in catalytic peroxymonosulfate activation for phenol degradation: variation of surface functionalities[J]. RSC Advances, 2019, 9(2): 974-983. |
| 25 | HU P D, SU H R, CHEN Z Y, et al. Selective degradation of organic pollutants using an efficient metal-free catalyst derived from carbonized polypyrrole via peroxymonosulfate activation[J]. Environmental Science and Technology, 2017, 51(19): 11288-11296. |
| 26 | HAN C, DUAN X G, ZHANG M J, et al. Role of electronic properties in partition of radical and nonradical processes of carbocatalysis toward peroxymonosulfate activation[J]. Carbon, 2019, 153: 73-80. |
| 27 | LEE H, LEE H J, JEONG J, et al. Activation of persulfates by carbon nanotubes: oxidation of organic compounds by nonradical mechanism[J]. Chemical Engineering Journal, 2015, 266: 28-33. |
| 28 | TANG L, LIU Y N, WANG J J, et al. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: electron transfer mechanism[J]. Applied Catalysis B: Environmental, 2018, 231: 1-10. |
| 29 | DUAN X G, O'DONNELL K, SUN H Q, et al. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions[J]. Small, 2015, 11(25): 3036-3044. |
| 30 | YUN E T, MOON G H, LEE H, et al. Oxidation of organic pollutants by peroxymonosulfate activated with low-temperature-modified nanodiamonds: understanding the reaction kinetics and mechanism[J]. Applied Catalysis B: Environmental, 2018, 237: 432-441. |
| 31 | WANG X B, TANG P, DING C, et al. Simultaneous enhancement of adsorption and peroxymonosulfate activation of nitrogen-doped reduced graphene oxide for bisphenol A removal[J]. Journal of Environmental Chemical Engineering, 2017, 5(5): 4291-4297. |
| 32 | WANG J, DUAN X G, DONG Q, et al. Facile synthesis of N-doped 3D graphene aerogel and its excellent performance in catalytic degradation of antibiotic contaminants in water[J]. Carbon, 2019, 144: 781-790. |
| 33 | LI D G, DUAN X G, SUN H Q, et al. Facile synthesis of nitrogen-doped graphene via low-temperature pyrolysis: the effects of precursors and annealing ambience on metal-free catalytic oxidation[J]. Carbon, 2017, 115: 649-658. |
| 34 | DUAN X G, AO Z M, LI D G, et al. Surface-tailored nanodiamonds as excellent metal-free catalysts for organic oxidation[J]. Carbon, 2016, 103: 404-411. |
| 35 | CHEN X, DUAN X G, OH W D, et al. Insights into nitrogen and boron-co-doped graphene toward high-performance peroxymonosulfate activation: maneuverable N-B bonding configurations and oxidation pathways[J]. Applied Catalysis B: Environmental, 2019, 253: 419-432. |
| 36 | PAN X X, CHEN J, WU N N, et al. Degradation of aqueous 2,4,4″-trihydroxybenzophenone by persulfate activated with nitrogen doped carbonaceous materials and the formation of dimer products[J]. Water Research, 2018, 143: 176-187. |
| 37 | SUN H Q, KWAN C K, SUVOROVA A, et al. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals[J]. Applied Catalysis B: Environmental, 2014, 154/155: 134-141. |
| [1] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [2] | WANG Jingang, ZHANG Jianbo, TANG Xuejiao, LIU Jinpeng, JU Meiting. Research progress on modification of Cu-SSZ-13 catalyst for denitration of automobile exhaust gas [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4636-4648. |
| [3] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [4] | BAI Zhihua, ZHANG Jun. Oxidative removal of NO in DTPMPA/Fenton system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4967-4973. |
| [5] | ZHANG Yaojie, ZHANG Chuanxiang, SUN Yue, ZENG Huihui, JIA Jianbo, JIANG Zhendong. Application of coal-based graphene quantum dots in supercapacitors [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4340-4350. |
| [6] | CHU Tiantian, LIU Runzhu, DU Gaohua, MA Jiahao, ZHANG Xiao’a, WANG Chengzhong, ZHANG Junying. Preparation and chemical degradability of organoguanidine-catalyzed dehydrogenation type RTV silicone rubbers [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3664-3673. |
| [7] | XU Peiyao, CHEN Biaoqi, KANKALA Ranjith Kumar, WANG Shibin, CHEN Aizheng. Research progress of nanomaterials for synergistic ferroptosis anticancer therapy [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3684-3694. |
| [8] | LI Yanling, ZHUO Zhen, CHI Liang, CHEN Xi, SUN Tanglei, LIU Peng, LEI Tingzhou. Research progress on preparation and application of nitrogen-doped biochar [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3720-3735. |
| [9] | XU Wei, LI Kaijun, SONG Linye, ZHANG Xinghui, YAO Shunhua. Research progress of photocatalysis and co-electrochemical degradation of VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3520-3531. |
| [10] | GONG Pengcheng, YAN Qun, CHEN Jinfu, WEN Junyu, SU Xiaojie. Properties and mechanism of eriochrome black T degradation by carbon nanotube-cobalt ferrite composites activated persulfate [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3572-3581. |
| [11] | YANG Jiatian, TANG Jinming, LIANG Zirong, LI Yinhong, HU Huayu, CHEN Yuan. Preparation and application of novel starch-based super absorbent polymer dust suppressant [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3187-3196. |
| [12] | WANG Jiuheng, RONG Nai, LIU Kaiwei, HAN Long, SHUI Taotao, WU Yan, MU Zhengyong, LIAO Xuqing, MENG Wenjia. Enhanced CO2 capture performance and strength of cellulose-templated CaO-based pellets with steam reactivation [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3217-3225. |
| [13] | WU Fengzhen, LIU Zhiwei, XIE Wenjie, YOU Yating, LAI Rouqiong, CHEN Yandan, LIN Guanfeng, LU Beili. Preparation of biomass derived Fe/N co-doped porous carbon and its application for catalytic degradation of Rhodamine B via peroxymonosulfate activation [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3292-3301. |
| [14] | YANG Hongmei, GAO Tao, YU Tao, QU Chengtun, GAO Jiapeng. Treatment of refractory organics sulfonated phenolic resin with ferrate [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3302-3308. |
| [15] | HE Chuan, WU Guoxun, LI Ang, ZHANG Fajie, BIAN Zijun, LU Chengzheng, WANG Lipeng, ZHAO Min. Characteristics of calcium and magnesium deactivation and regeneration of waste incineration SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2413-2420. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||