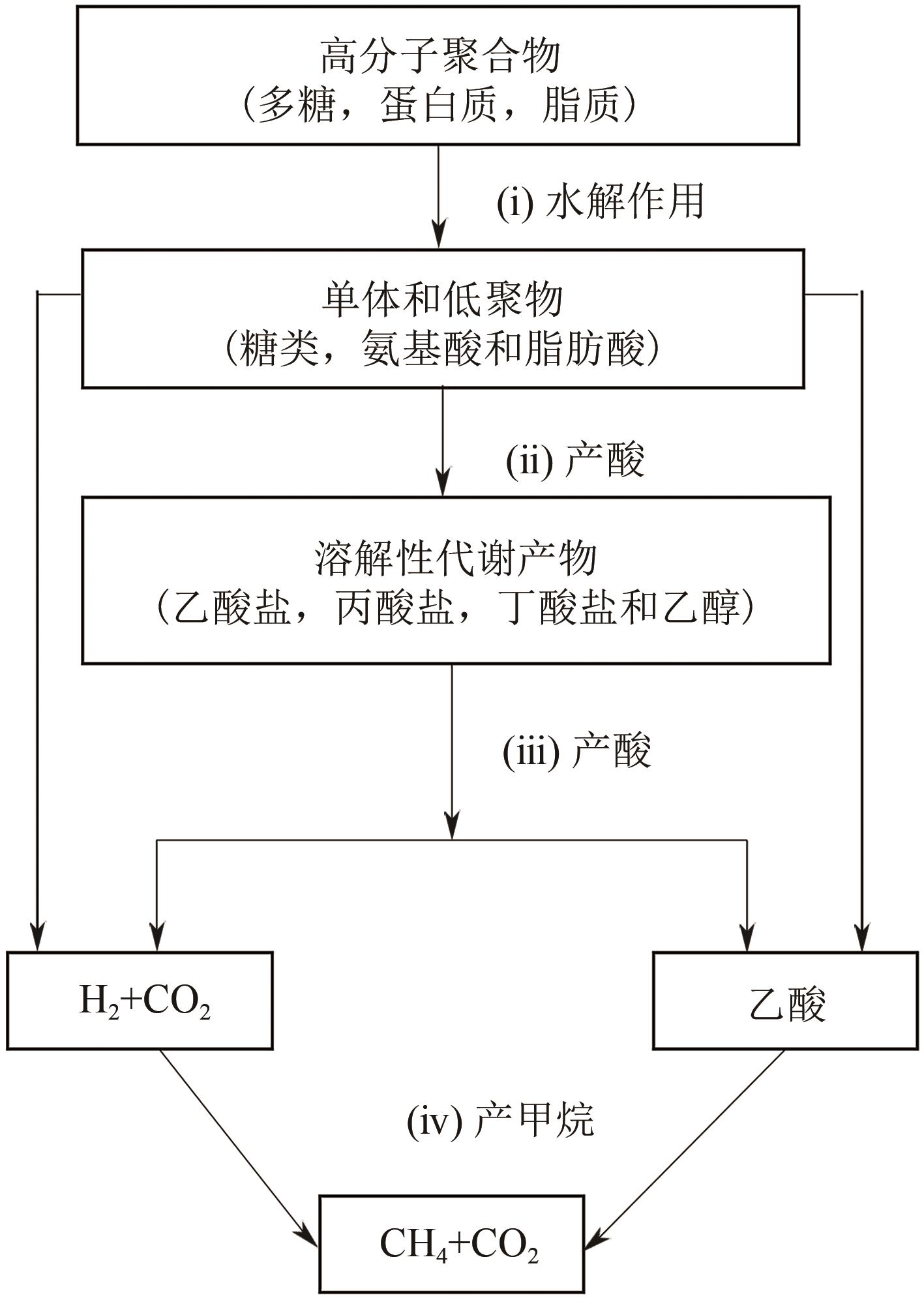
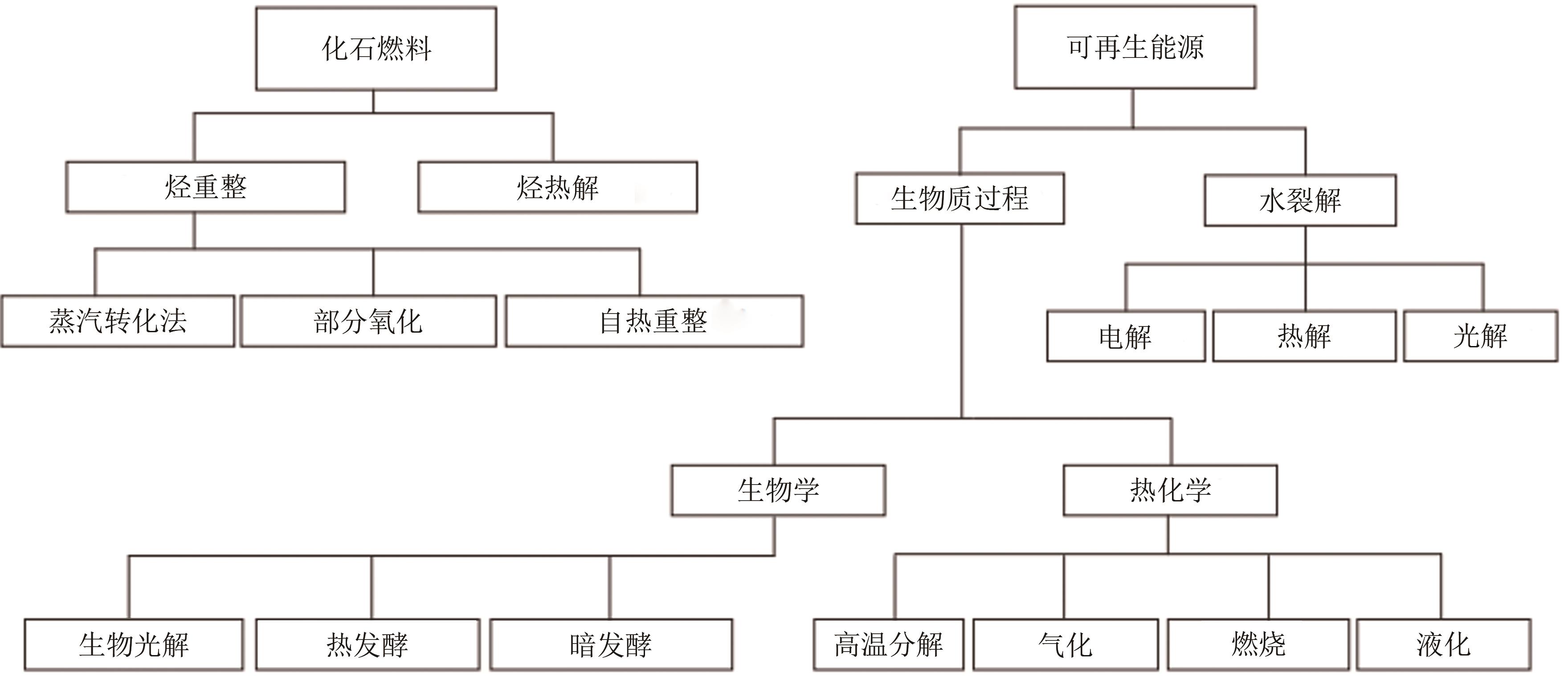
| 1 |
HÖÖK M, TANG X. Depletion of fossil fuels and anthropogenic climate change—a review[J]. Energy Policy, 2013, 52: 797-809.
|
| 2 |
RAGAUSKAS A J, WILLIAMS C K, DAVISON B H, et al. The path forward for biofuels and biomaterials[J]. Science, 2006, 311: 484-489.
|
| 3 |
MARTIN M R, FORNERO J J, STARK R, et al. A single-culture bioprocess of methanothermobacter thermautotrophicus to upgrade digester biogas by CO2 -to-CH4 conversion with H2[J]. Archaea, 2013, 2013: 1-11.
|
| 4 |
BASSANI I, KOUGIAS P G, TREU L, et al. Optimization of hydrogen dispersion in thermophilic up-flow reactors for ex situ biogas upgrading[J]. Bioresour Technol, 2017, 234: 310-319.
|
| 5 |
HU Y, HAO X, ZHAO D, et al. Enhancing the CH4 yield of anaerobic digestion via endogenous CO2 fixation by exogenous H2[J]. Chemosphere, 2015, 140: 34-39.
|
| 6 |
STEVEN D A, MARTINY J B H. Resistance, resilience, and redundancy in microbial communities[J]. PANS, 2008, 105: 11512-11519.
|
| 7 |
SAKAI S, IMACHI H, HANADA S, et al. Methanocella paludicola gen. nov., sp. nov., a methane-producing archaeon, the first isolate of the lineage ‘Rice Cluster I’, and proposal of the new archaeal order Methanocellales ord. nov[J]. International Journal of Systematic and Evolutionary Microbiology, 2008, 58(Pt 4): 929-936.
|
| 8 |
PAUL K, NONOH J O, MIKULSKI L, et al. “Methanoplasmatales,” thermoplasmatales-related archaea in termite guts and other environments, are the seventh order of methanogens[J]. Applied and Environmental Microbiology, 2012, 78(23): 8245-8253.
|
| 9 |
GARCIA J L, OLLIVIER B, WHITMAN W B. The order methanomicrobiales[M]. New York: Springer, 2006: 231-243.
|
| 10 |
ZABRANSKA J, POKORNA D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens[J]. Biotechnol. Advance, 2018, 36(3): 707-20.
|
| 11 |
BONIN A S, BOONE D R. The order methanobacteriales[M]. New York: Springer, 2006: 231-243.
|
| 12 |
WHITMAN W B, JEANTHON C. Methanococcales[M]. New York: Springer, 2006: 257-273.
|
| 13 |
KURR M, HUBER R, KONIG H, et al. Methanopyrus-kandleri, gen and sp-nov represents a novel group of hyperthermophilic methanogens, growing at 110℃[J]. Archives of Microbiology, 1991, 156(4): 239-247.
|
| 14 |
SLESAREV A I, MEZHEVAYA K V, MAKAROVA K S, et al. The complete genome of hyperthermophile Methanopyrus kandleri AV19 and monophyly of archaeal methanogens[J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(7): 4644-4649.
|
| 15 |
LUO G, JOHANSSON S, BOE K, et al. Simultaneous hydrogen utilization and in situ biogas upgrading in an anaerobic reactor[J]. Biotechnol. Bioeng., 2012, 109(4): 1088-1094.
|
| 16 |
BALAT M. Potential importance of hydrogen as a future solution to environmental and transportation problems[J]. International Journal of Hydrogen Energy, 2008, 33(15): 4013-4029.
|
| 17 |
BAGI Z, ACS N, BALINT B, et al. Biotechnological intensification of biogas production[J]. Applied Microbiology and Biotechnology, 2007, 76(2): 473-482.
|
| 18 |
NIKOLAIDIS P, POULLIKKAS A. A comparative overview of hydrogen production processes[J]. Renewable and Sustainable Energy Reviews, 2017, 67: 597-611.
|
| 19 |
BALAT H, KRTAY E. Hydrogen from biomass-present scenario and future prospects[J]. International Journal of Hydrogen Energy, 2010, 35(14): 7416-7426.
|
| 20 |
PARTHASARATHY P, NARAYANAN K S. Hydrogen production from steam gasification of biomass: influence of process parameters on hydrogen yield-a review[J]. Renewable Energy, 2014, 66: 570-579.
|
| 21 |
DEMIRBASß A. Biomass resource facilities and biomass conversion processing for fuels and chemicals[J]. Energy Conversion and Management, 2001, 42: 1357-78.
|
| 22 |
MCKENDRY P. Energy production from biomass (part 1): overview of biomass[J]. Bioresource Technology, 2002, 83: 37-46.
|
| 23 |
ZHANG Y, YING Z, ZHOU J, et al. Electrolysis of the bunsen reaction and properties of the membrane in the sulfuriodine thermochemical cycle[J]. Industrial & Engineering Chemistry Research, 2014, 53(35): 13581-13588.
|
| 24 |
ROSSMEISL J, LOGADOTTIR A, NØRSKOV J K. Electrolysis of water on (oxidized) metal surfaces[J]. Chemical Physics, 2005, 319(1/2/3): 178-184.
|
| 25 |
BLOK K. Enhanced policies for the improvement of electricity efficiencies[J]. Energy Policy, 2005, 33(13): 1635-1641.
|
| 26 |
ĆOSIĆ B, KRAJAČIĆ G, DUIĆ N. A 100% renewable energy system in the year 2050: the case of macedonia[J]. Energy, 2012, 48(1): 80-87.
|
| 27 |
郗凌霄, 姚立国. 氢气的来源及应用[J]. 精细与专用化学品, 2017, 25(10): 42-45.
|
|
CHI L X, YAO L G. The sources and application of hydrogen[J]. Fine and Specialty Chemical, 2017, 25(10): 42-45.
|
| 28 |
WANG W, XIE L, LUO G, et al. Performance and microbial community analysis of the anaerobic reactor with coke oven gas biomethanation and in situ biogas upgrading [J]. Bioresource Technology, 2013, 146: 234-239.
|
| 29 |
CONNAUGHTON S, COLLINS G, O’FLAHERTY V. Psychrophilic and mesophilic anaerobic digestion of brewery effluent: a comparative study[J]. Water Research, 2006, 40(13): 2503-2510.
|
| 30 |
GUNNIGLE E, NIELSEN J L, FUSZARD M, et al. Functional responses and adaptation of mesophilic microbial communities to psychrophilic anaerobic digestion[J]. FEMS Microbiol. Ecol., 2015, 91(12): 1-15
|
| 31 |
O’REILLY J, LEE C, COLLINS G, et al. Quantitative and qualitative analysis of methanogenic communities in mesophilically and psychrophilically cultivated anaerobic granular biofilims[J]. Water Research, 2009, 43(14): 3365-3374.
|
| 32 |
JU F, ZHANG T. Novel microbial populations in ambient and mesophilic biogas-producing and phenol-degrading consortia unraveled by high-throughput sequencing[J]. Microbial Ecology, 2014, 68(2): 235-246.
|
| 33 |
BASSANI I, KOUGIAS P G, TREU L, et al. Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions[J]. Environmental Science & Technology, 2015, 49(20): 12585-12593.
|
| 34 |
LEE J C, KIM J H, CHANG W S, et al. Biological conversion of CO2 to CH4 using hydrogenotrophic methanogen in a fixed bed reactor[J]. Journal of Chemical Technology & Biotechnology, 2012, 87(6): 844-847.
|
| 35 |
BARANSI-KARKABY K, HASSANIN M, MUHSEIN S, et al. Innovative ex-situ biological biogas upgrading using immobilized biomethanation bioreactor (IBBR)[J]. Water Science and Technology, 2020, 81(6): 1319-1328.
|
| 36 |
ZHAO L, WANG Z, REN H Y, et al. Improving biogas upgrading and liquid chemicals production simultaneously by a membrane biofilm reactor[J]. Bioresource Technology, 2020, 313: 123693.
|
| 37 |
LUO G, ANGELIDAKI I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture[J]. Biotechnol. Bioeng., 2012, 109(11): 2729-2736.
|
| 38 |
CORBELLINI V, KOUGIAS P G, TREU L, et al. Hybrid biogas upgrading in a two-stage thermophilic reactor[J]. Energy Conversion and Management, 2018, 168: 1-10.
|
| 39 |
PORTÉ H, KOUGIAS P G, ALFARO N, et al. Process performance and microbial community structure in thermophilic trickling biofilter reactors for biogas upgrading[J]. Science of the Total Environment, 2019, 655: 529-538.
|
| 40 |
DONG N, BU F, ZHOU Q, et al. Performance and microbial community of hydrogenotrophic methanogenesis under thermophilic and extreme-thermophilic conditions[J]. Bioresource Technology, 2018, 266: 454-462.
|
| 41 |
RACHBAUER L, VOITL G, BOCHMANN G, et al. Biological biogas upgrading capacity of a hydrogenotrophic community in a trickle-bed reactor[J]. Applied Energy, 2016, 180: 483-490.
|
| 42 |
JU D H, SHIN J H, LEE H K, et al. Effects of pH conditions on the biological conversion of carbon dioxide to methane in a hollow-fiber membrane biofilm reactor (Hf-MBfR)[J]. Desalination, 2008, 234(1/2/3): 409-415.
|
| 43 |
KOUGIAS P G, TREU L, BENAVENTE D P, et al. Ex-situ biogas upgrading and enhancement in different reactor systems[J]. Bioresource Technology, 2017, 225: 429-437.
|
| 44 |
VOELKLEIN M A, RUSMANIS D, MURPHY J D. Biological methanation: strategies for in-situ and ex-situ upgrading in anaerobic digestion[J]. Applied Energy, 2019, 235: 1061-1071.
|
| 45 |
STREVETT K A, VIETH R F, GRASSO D. Chemo-autotrophic biogas purification for methane enrichment[J]. Chemical Engineering Journal & the Biochemical Engineering Journal, 1995, 58(1): 71-79.
|
| 46 |
AHRING B K. Status on science and application of thermophilic anaerobic digestion[J]. Water Science and Technology, 1994, 30(12): 241-249.
|
| 47 |
AHRING B K, IBRAHIM A A, MLADENOVSKA Z. Effect of temperature increase from 55℃ to 65℃ on performance and microbial population dynamics of an anaerobic reactor treating cattle manure[J]. Water Research, 2001, 35(10): 2446-2452.
|
| 48 |
PAP B, GYORKEI A, BOBOESCU I Z, et al. Temperature-dependent transformation of biogas-producing microbial communities points to the increased importance of hydrogenotrophic methanogenesis under thermophilic operation[J]. Bioresource Technology, 2015, 177: 375-380.
|
| 49 |
KOHRS F, HEYER R, MAGNUSSEN A, et al. Sample prefractionation with liquid isoelectric focusing enables in depth microbial metaproteome analysis of mesophilic and thermophilic biogas plants[J]. Anaerobe, 2014, 29: 59-67.
|
| 50 |
LYU Z, WU X, ZHOU B, et al. Effect of one step temperature increment from mesophilic to thermophilic anaerobic digestion on the linked pattern between bacterial and methanogenic communities[J]. Bioresource Technology, 2019, 292: 121968.
|
| 51 |
MCKEOWN R M, SCULLY C, ENRIGHT A M, et al. Psychrophilic methanogenic community development during long-term cultivation of anaerobic granular biofilms[J]. ISME Journal, 2009, 3(11): 1231-1242.
|
| 52 |
REGUEIRO L, CARBALLA M, LEMA J M. Outlining microbial community dynamics during temperature drop and subsequent recovery period in anaerobic co-digestion systems[J]. Journal of Biotechnology, 2014, 192(Pt A): 179-186.
|
| 53 |
BIALEK K, KUMAR A, MAHONY T, et al. Microbial community structure and dynamics in anaerobic fluidized-bed and granular sludge-bed reactors: influence of operational temperature and reactor configuration[J]. Microbial Biotechnology, 2012, 5(6): 738-752.
|
| 54 |
SINGH L, ALAM S I, RAMANA K V. Effect of fluctuating temperature regime on psychrophilic anaerobic digestion of nightsoil[J]. Defence Science Journal, 1999, 49(2): 135-140.
|
| 55 |
CHO K, LEE J, KIM W, et al. Behavior of methanogens during start-up of farm-scale anaerobic digester treating swine wastewater[J]. Process Biochemistry, 2013, 48(9): 1441-1445.
|