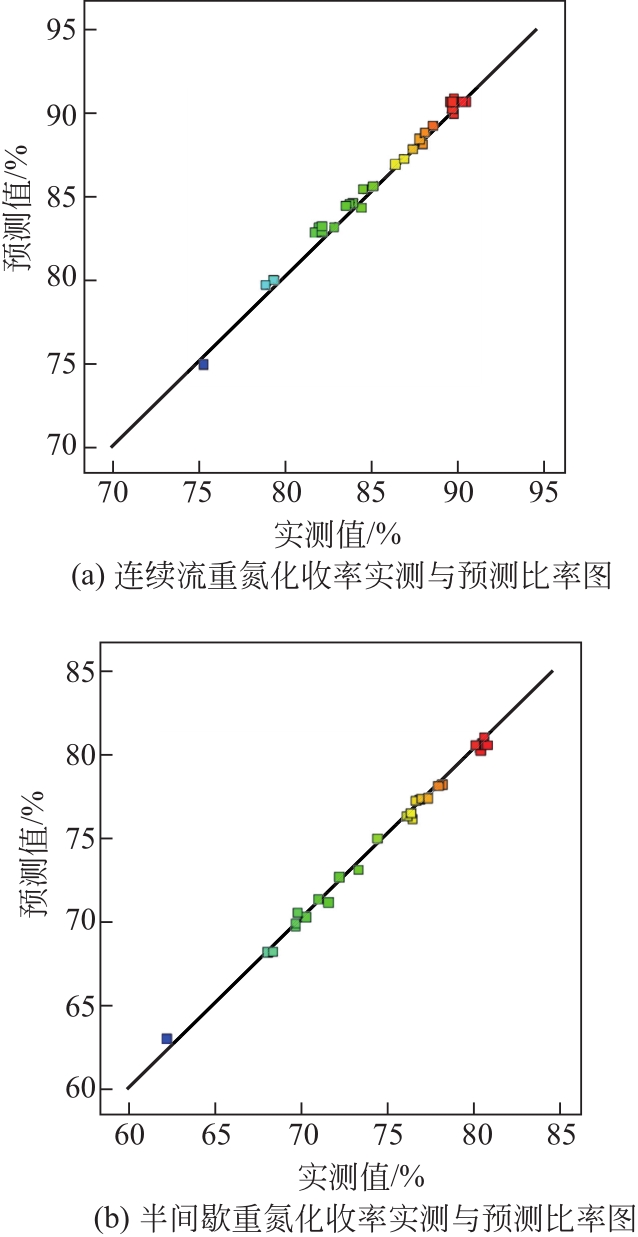
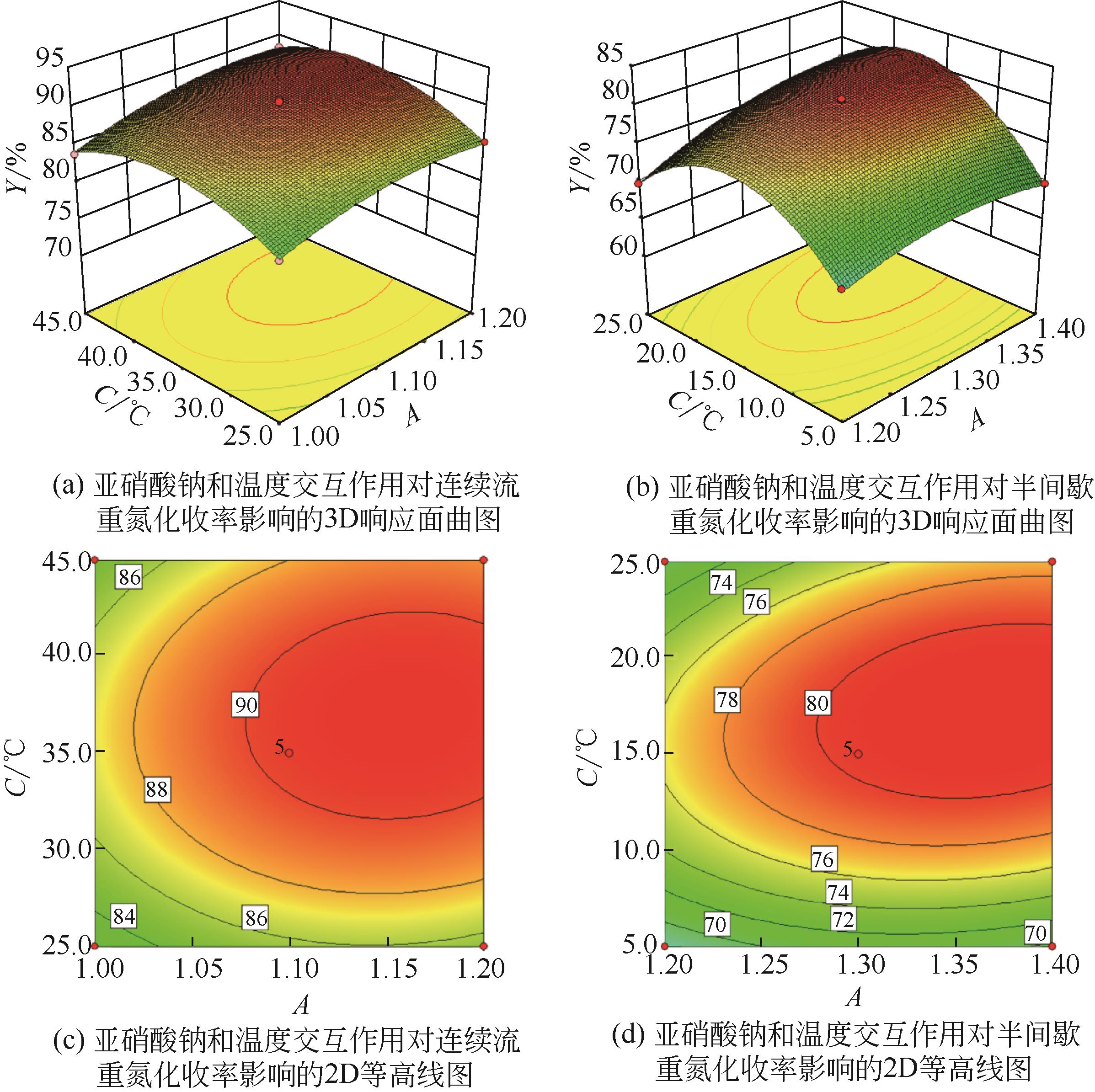
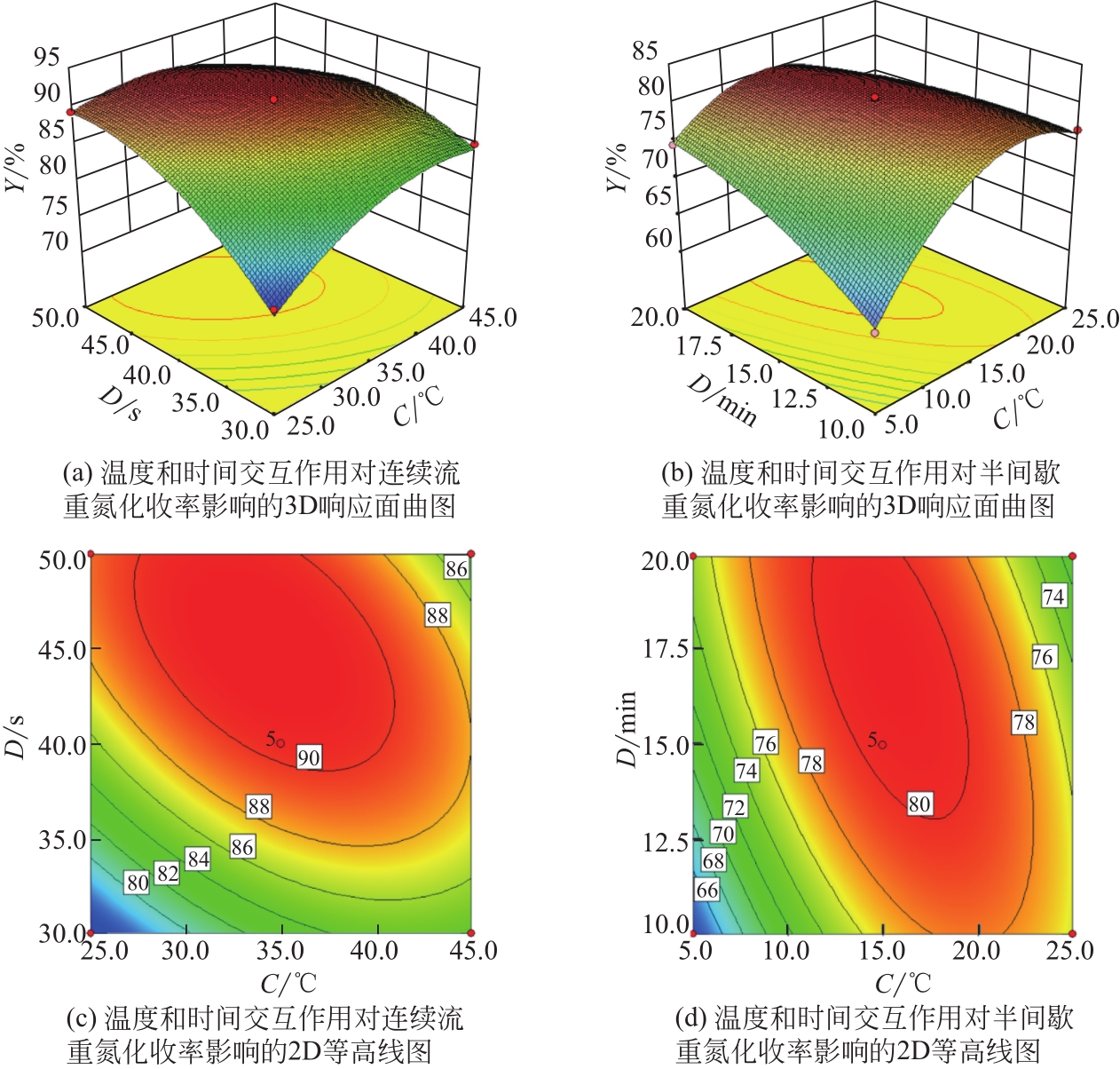
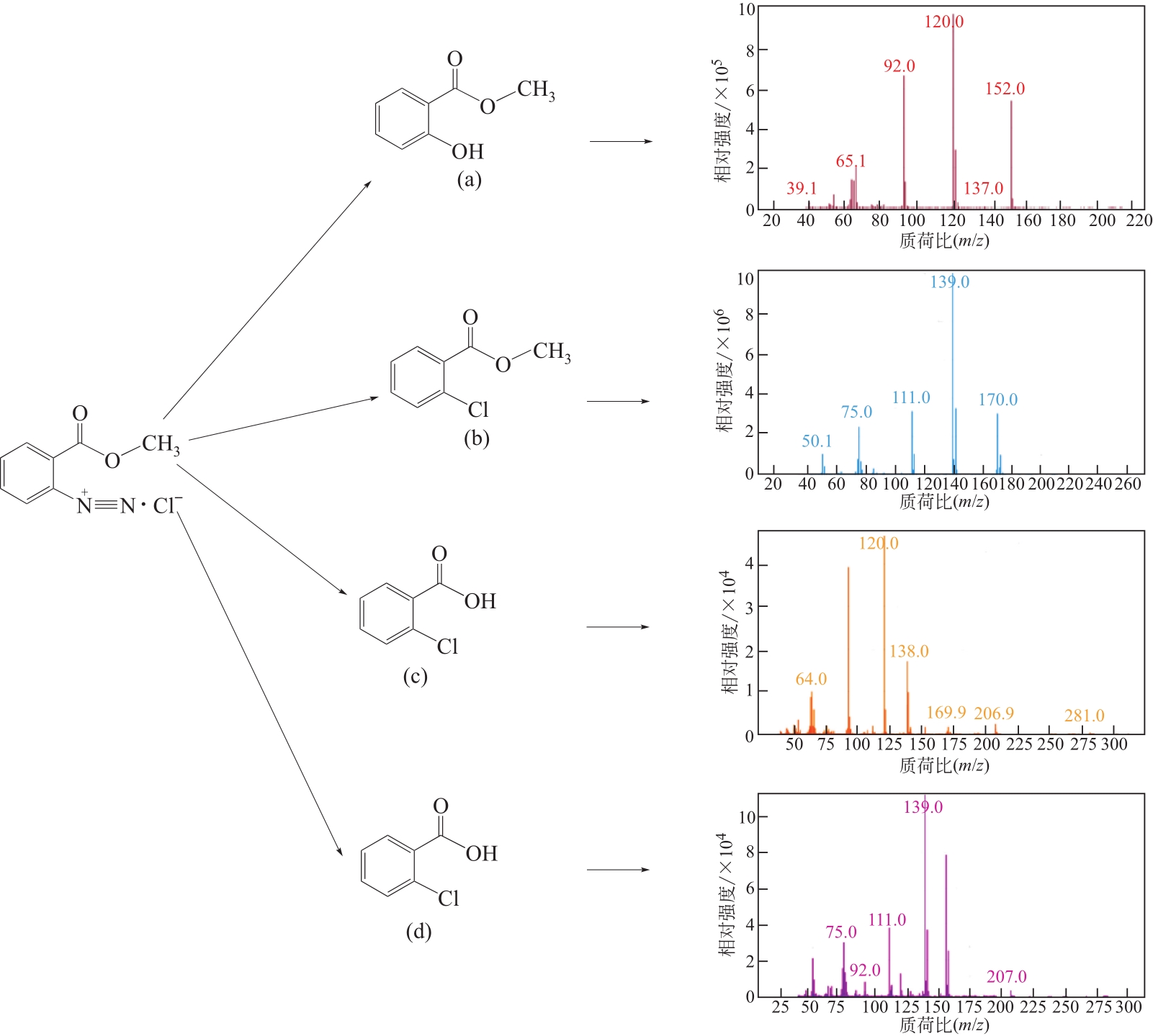
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (10): 5678-5691.DOI: 10.16085/j.issn.1000-6613.2020-2203
• Fine chemicals • Previous Articles Next Articles
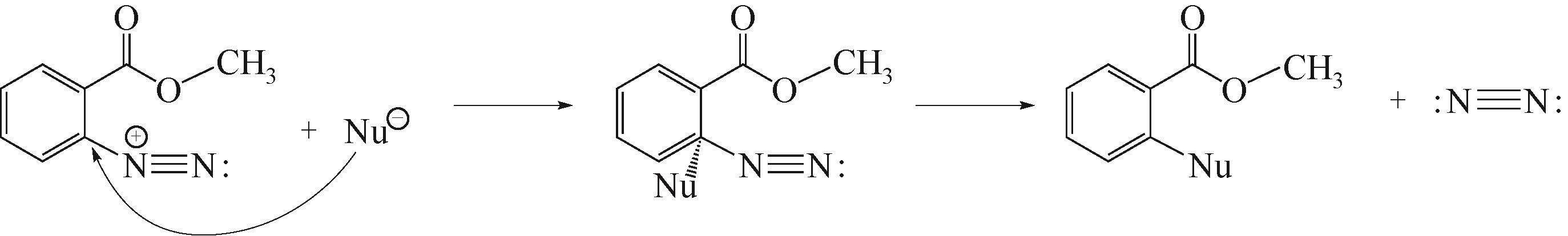
Continuous-flow diazotization of methyl anthranilate in microreactor system
WANG Ben1( ), WANG Chao1, YIN Jinhua2(
), WANG Chao1, YIN Jinhua2( )
)
- 1.College of Environment and Safety Engineering, Qingdao University of Science and Technology, Qingdao 266042, Shandong, China
2.College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266042, Shandong, China
-
Received:2020-11-04Revised:2020-12-22Online:2021-10-25Published:2021-10-10 -
Contact:YIN Jinhua
微反应器内邻氨基苯甲酸甲酯的连续重氮化工艺
- 1.青岛科技大学环境与安全工程学院,山东 青岛 266042
2.青岛科技大学化工学院,山东 青岛 266042
-
通讯作者:尹进华 -
作者简介:王犇(1970—),男,博士,副教授,硕士生导师,研究方向为安全科学、化工过程强化。E-mail:wb1970@163.com 。 -
基金资助:山东省R&D重点项目基金(GG201809250363);山东省重点研发计划(2019GGX104014)
CLC Number:
Cite this article
WANG Ben, WANG Chao, YIN Jinhua. Continuous-flow diazotization of methyl anthranilate in microreactor system[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5678-5691.
王犇, 王超, 尹进华. 微反应器内邻氨基苯甲酸甲酯的连续重氮化工艺[J]. 化工进展, 2021, 40(10): 5678-5691.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-2203
| 水平 | 因素 | |||
|---|---|---|---|---|
| A | B | C | D | |
| -1 | 1∶1.0 | 1∶2.4 | 25℃ | 30s |
| 0 | 1∶1.1 | 1∶2.6 | 35℃ | 40s |
| 1 | 1∶1.2 | 1∶2.8 | 45℃ | 50s |
| 水平 | 因素 | |||
|---|---|---|---|---|
| A | B | C | D | |
| -1 | 1∶1.0 | 1∶2.4 | 25℃ | 30s |
| 0 | 1∶1.1 | 1∶2.6 | 35℃ | 40s |
| 1 | 1∶1.2 | 1∶2.8 | 45℃ | 50s |
| 水平 | 因素 | |||
|---|---|---|---|---|
| A | B | C | D | |
| -1 | 1∶1.2 | 1∶2.8 | 5℃ | 10min |
| 0 | 1∶1.3 | 1∶3.0 | 15℃ | 15min |
| 1 | 1∶1.4 | 1∶3.2 | 25℃ | 20min |
| 水平 | 因素 | |||
|---|---|---|---|---|
| A | B | C | D | |
| -1 | 1∶1.2 | 1∶2.8 | 5℃ | 10min |
| 0 | 1∶1.3 | 1∶3.0 | 15℃ | 15min |
| 1 | 1∶1.4 | 1∶3.2 | 25℃ | 20min |
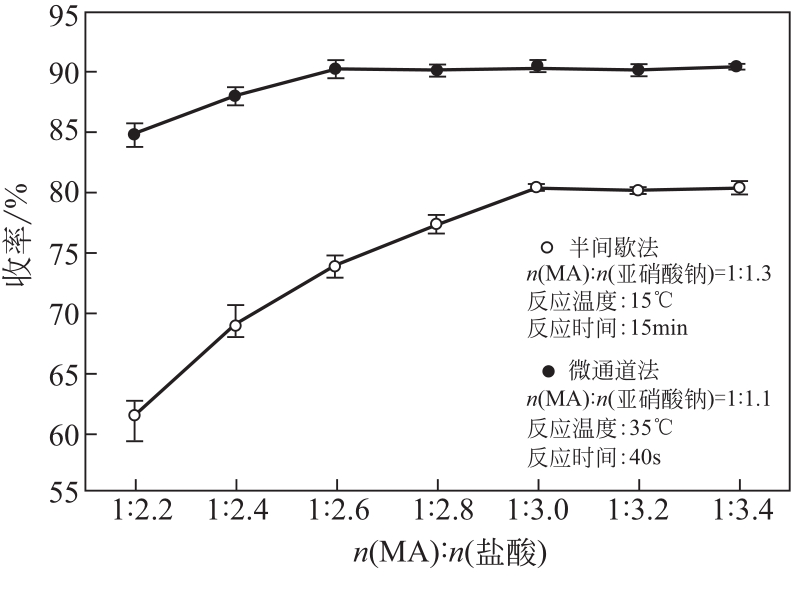
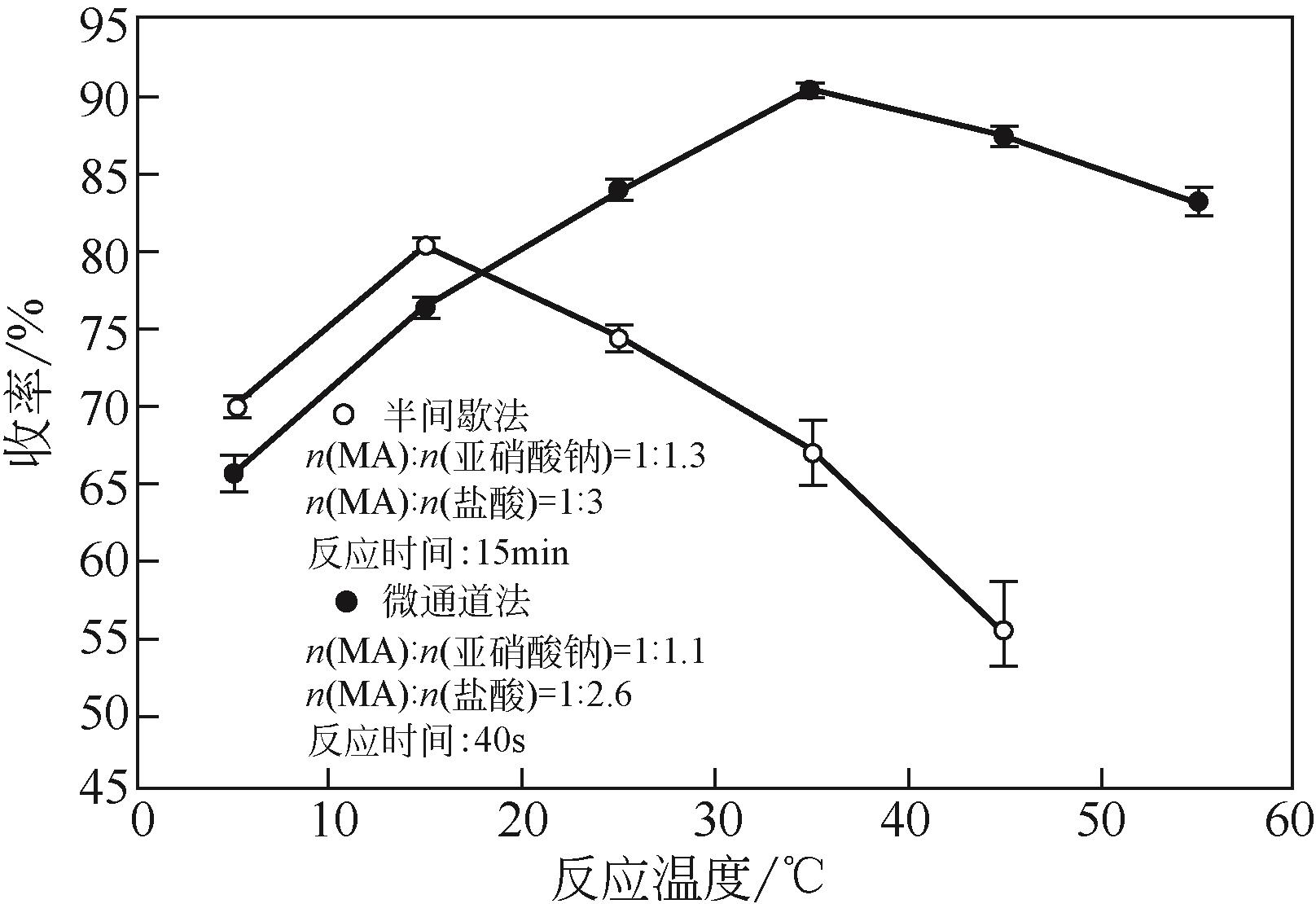
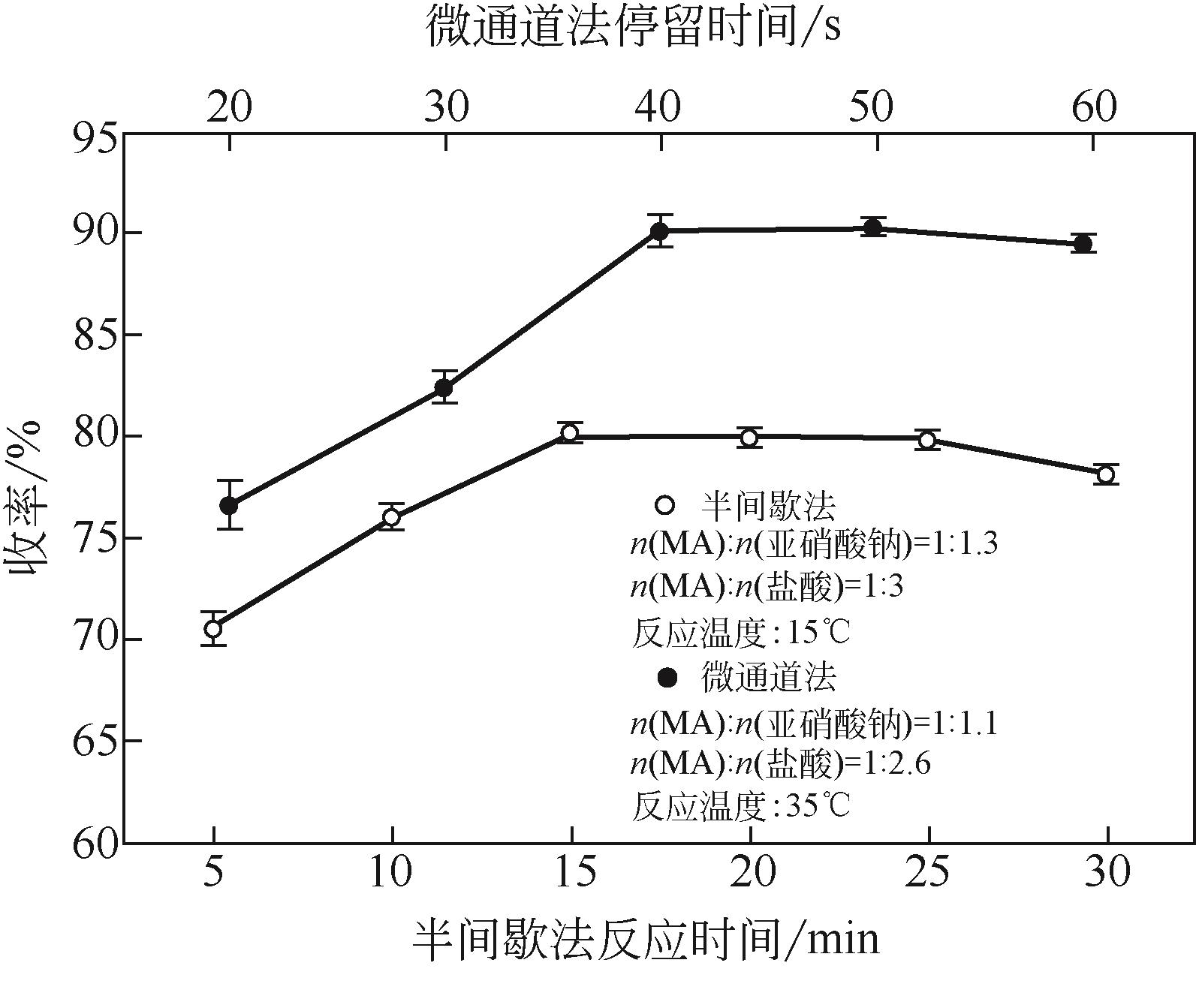
| 编号 | 因素编码值 | 连续收率/% | 半间歇收率/% | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | 实测 | 预测 | 实测 | 预测 | |
| 1 | 0 | 0 | 0 | 0 | 90.7 | 90.40 | 80.5 | 80.44 |
| 2 | -11 | 0 | -11 | 0 | 82.5 | 82.66 | 68.5 | 68.11 |
| 3 | -11 | 1 | 0 | 0 | 87.8 | 87.58 | 78.0 | 77.98 |
| 4 | 1 | -11 | 0 | 0 | 88.4 | 87.88 | 78.2 | 78.08 |
| 5 | 0 | 0 | -11 | -11 | 75.6 | 74.77 | 62.4 | 62.92 |
| 6 | 0 | 0 | 1 | 1 | 84.3 | 84.40 | 71.7 | 71.05 |
| 7 | -11 | -11 | 0 | 0 | 83.2 | 82.95 | 71.1 | 71.25 |
| 8 | -11 | 0 | 0 | 1 | 86.8 | 86.68 | 76.2 | 76.20 |
| 9 | 0 | 0 | 0 | 0 | 90.0 | 90.40 | 80.1 | 80.44 |
| 10 | -11 | 0 | 1 | 0 | 83.9 | 84.23 | 69.8 | 69.65 |
| 11 | 0 | 1 | 1 | 0 | 88.2 | 88.21 | 76.7 | 77.10 |
| 12 | 0 | -11 | 0 | -11 | 79.2 | 79.51 | 73.4 | 72.98 |
| 13 | 1 | 0 | -11 | 0 | 85.5 | 85.39 | 69.8 | 69.80 |
| 14 | 1 | 0 | 0 | -11 | 82.3 | 82.94 | 76.9 | 77.19 |
| 15 | 1 | 1 | 0 | 0 | 90.2 | 89.72 | 80.4 | 80.12 |
| 16 | 0 | 1 | 0 | 1 | 90.1 | 90.01 | 80.6 | 80.86 |
| 17 | -11 | 0 | 0 | -11 | 79.7 | 79.81 | 72.3 | 72.55 |
| 18 | 0 | 1 | 0 | -11 | 82.5 | 82.99 | 77.4 | 77.26 |
| 19 | 0 | 0 | -11 | 1 | 89.0 | 88.98 | 74.5 | 74.87 |
| 20 | 1 | 0 | 1 | 0 | 88.5 | 88.56 | 77.0 | 77.23 |
| 21 | 0 | 0 | 0 | 0 | 90.9 | 90.40 | 80.1 | 80.44 |
| 22 | 0 | -11 | -11 | 0 | 82.1 | 82.61 | 68.2 | 68.09 |
| 23 | 0 | -11 | 0 | 1 | 87.3 | 87.03 | 76.4 | 76.38 |
| 24 | 0 | 0 | 0 | 0 | 90.1 | 90.40 | 80.7 | 80.44 |
| 25 | 0 | 0 | 1 | -11 | 84.8 | 84.08 | 76.5 | 76.00 |
| 26 | 0 | -11 | 1 | 0 | 84.1 | 84.33 | 69.9 | 70.42 |
| 27 | 0 | 0 | 0 | 0 | 90.3 | 90.40 | 80.8 | 80.44 |
| 28 | 0 | 1 | -11 | 0 | 84.9 | 85.19 | 70.4 | 70.17 |
| 29 | 1 | 0 | 0 | 1 | 90.2 | 90.61 | 80.5 | 80.54 |
| 编号 | 因素编码值 | 连续收率/% | 半间歇收率/% | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | 实测 | 预测 | 实测 | 预测 | |
| 1 | 0 | 0 | 0 | 0 | 90.7 | 90.40 | 80.5 | 80.44 |
| 2 | -11 | 0 | -11 | 0 | 82.5 | 82.66 | 68.5 | 68.11 |
| 3 | -11 | 1 | 0 | 0 | 87.8 | 87.58 | 78.0 | 77.98 |
| 4 | 1 | -11 | 0 | 0 | 88.4 | 87.88 | 78.2 | 78.08 |
| 5 | 0 | 0 | -11 | -11 | 75.6 | 74.77 | 62.4 | 62.92 |
| 6 | 0 | 0 | 1 | 1 | 84.3 | 84.40 | 71.7 | 71.05 |
| 7 | -11 | -11 | 0 | 0 | 83.2 | 82.95 | 71.1 | 71.25 |
| 8 | -11 | 0 | 0 | 1 | 86.8 | 86.68 | 76.2 | 76.20 |
| 9 | 0 | 0 | 0 | 0 | 90.0 | 90.40 | 80.1 | 80.44 |
| 10 | -11 | 0 | 1 | 0 | 83.9 | 84.23 | 69.8 | 69.65 |
| 11 | 0 | 1 | 1 | 0 | 88.2 | 88.21 | 76.7 | 77.10 |
| 12 | 0 | -11 | 0 | -11 | 79.2 | 79.51 | 73.4 | 72.98 |
| 13 | 1 | 0 | -11 | 0 | 85.5 | 85.39 | 69.8 | 69.80 |
| 14 | 1 | 0 | 0 | -11 | 82.3 | 82.94 | 76.9 | 77.19 |
| 15 | 1 | 1 | 0 | 0 | 90.2 | 89.72 | 80.4 | 80.12 |
| 16 | 0 | 1 | 0 | 1 | 90.1 | 90.01 | 80.6 | 80.86 |
| 17 | -11 | 0 | 0 | -11 | 79.7 | 79.81 | 72.3 | 72.55 |
| 18 | 0 | 1 | 0 | -11 | 82.5 | 82.99 | 77.4 | 77.26 |
| 19 | 0 | 0 | -11 | 1 | 89.0 | 88.98 | 74.5 | 74.87 |
| 20 | 1 | 0 | 1 | 0 | 88.5 | 88.56 | 77.0 | 77.23 |
| 21 | 0 | 0 | 0 | 0 | 90.9 | 90.40 | 80.1 | 80.44 |
| 22 | 0 | -11 | -11 | 0 | 82.1 | 82.61 | 68.2 | 68.09 |
| 23 | 0 | -11 | 0 | 1 | 87.3 | 87.03 | 76.4 | 76.38 |
| 24 | 0 | 0 | 0 | 0 | 90.1 | 90.40 | 80.7 | 80.44 |
| 25 | 0 | 0 | 1 | -11 | 84.8 | 84.08 | 76.5 | 76.00 |
| 26 | 0 | -11 | 1 | 0 | 84.1 | 84.33 | 69.9 | 70.42 |
| 27 | 0 | 0 | 0 | 0 | 90.3 | 90.40 | 80.8 | 80.44 |
| 28 | 0 | 1 | -11 | 0 | 84.9 | 85.19 | 70.4 | 70.17 |
| 29 | 1 | 0 | 0 | 1 | 90.2 | 90.61 | 80.5 | 80.54 |
| 来源 | 平方和和 | 自由度 | 均方 | F值 | P值 |
|---|---|---|---|---|---|
| 模型 | 446.06 | 14 | 31.86 | 111.65 | <0.0001 |
| A | 37.45 | 1 | 37.45 | 131.25 | <0.0001 |
| B | 31.36 | 1 | 31.36 | 109.91 | <0.0001 |
| C | 16.80 | 1 | 16.80 | 58.89 | <0.0001 |
| D | 158.41 | 1 | 158.41 | 555.14 | <0.0001 |
| AB | 1.96 | 1 | 1.96 | 6.87 | 0.0201 |
| AC | 0.64 | 1 | 0.64 | 2.24 | 0.1564 |
| AD | 0.16 | 1 | 0.16 | 0.56 | 0.4664 |
| BC | 0.42 | 1 | 0.42 | 1.48 | 0.2438 |
| BD | 0.063 | 1 | 0.063 | 0.22 | 0.6470 |
| CD | 48.30 | 1 | 48.30 | 169.27 | <0.0001 |
| A2 | 17.04 | 1 | 17.04 | 59.72 | <0.0001 |
| B2 | 19.77 | 1 | 19.77 | 69.28 | <0.0001 |
| C2 | 82.71 | 1 | 82.71 | 289.84 | <0.0001 |
| D2 | 92.23 | 1 | 92.23 | 323.22 | <0.0001 |
| 残差 | 3.99 | 14 | 0.27 | ||
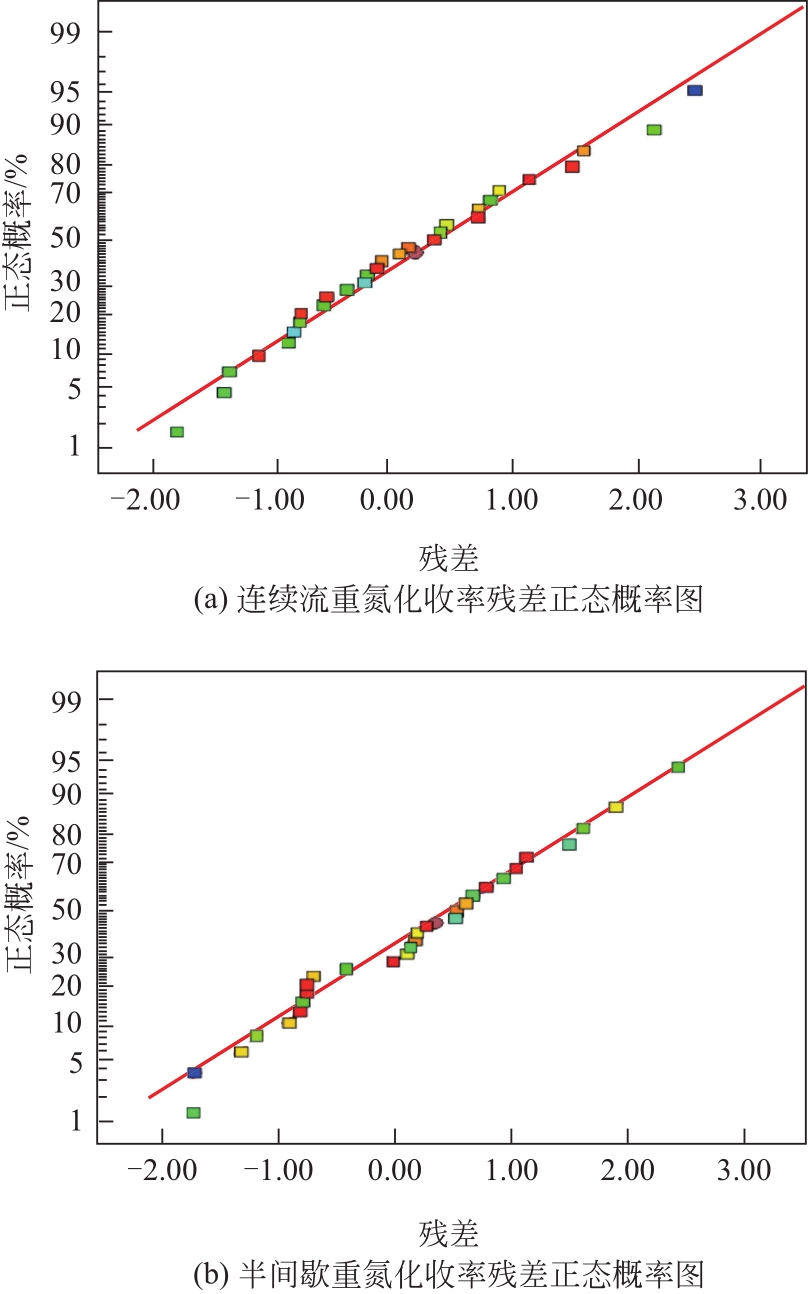
| 失拟误差 | 3.39 | 10 | 0.34 | 2.26 | 0.2240 |
| 纯误差 | 0.60 | 4 | 0.15 | ||
| 总误差 | 450.02 | 28 | |||
| 标准偏差 | 0.53 | 预测系数R | 0.9545 | ||
| 确定系数R2 | 0.9911 | 变异系数 | 0.62% | ||
| 调整系数R | 0.9822 | 精密度 | 41.234 | ||
| 来源 | 平方和和 | 自由度 | 均方 | F值 | P值 |
|---|---|---|---|---|---|
| 模型 | 446.06 | 14 | 31.86 | 111.65 | <0.0001 |
| A | 37.45 | 1 | 37.45 | 131.25 | <0.0001 |
| B | 31.36 | 1 | 31.36 | 109.91 | <0.0001 |
| C | 16.80 | 1 | 16.80 | 58.89 | <0.0001 |
| D | 158.41 | 1 | 158.41 | 555.14 | <0.0001 |
| AB | 1.96 | 1 | 1.96 | 6.87 | 0.0201 |
| AC | 0.64 | 1 | 0.64 | 2.24 | 0.1564 |
| AD | 0.16 | 1 | 0.16 | 0.56 | 0.4664 |
| BC | 0.42 | 1 | 0.42 | 1.48 | 0.2438 |
| BD | 0.063 | 1 | 0.063 | 0.22 | 0.6470 |
| CD | 48.30 | 1 | 48.30 | 169.27 | <0.0001 |
| A2 | 17.04 | 1 | 17.04 | 59.72 | <0.0001 |
| B2 | 19.77 | 1 | 19.77 | 69.28 | <0.0001 |
| C2 | 82.71 | 1 | 82.71 | 289.84 | <0.0001 |
| D2 | 92.23 | 1 | 92.23 | 323.22 | <0.0001 |
| 残差 | 3.99 | 14 | 0.27 | ||
| 失拟误差 | 3.39 | 10 | 0.34 | 2.26 | 0.2240 |
| 纯误差 | 0.60 | 4 | 0.15 | ||
| 总误差 | 450.02 | 28 | |||
| 标准偏差 | 0.53 | 预测系数R | 0.9545 | ||
| 确定系数R2 | 0.9911 | 变异系数 | 0.62% | ||
| 调整系数R | 0.9822 | 精密度 | 41.234 | ||
| 来源 | 平方和 | 自由度 | 均方 | F值 | P值 |
|---|---|---|---|---|---|
| 模型 | 661.71 | 14 | 47.26 | 239.50 | <0.0001 |
| A | 60.30 | 1 | 60.30 | 305.56 | <0.0001 |
| B | 57.64 | 1 | 57.64 | 292.08 | <0.0001 |
| C | 64.40 | 1 | 64.40 | 326.35 | <0.0001 |
| D | 36.75 | 1 | 36.75 | 186.22 | <0.0001 |
| AB | 5.52 | 1 | 5.52 | 27.98 | 0.0001 |
| AC | 8.70 | 1 | 8.70 | 44.10 | <0.0001 |
| AD | 0.023 | 1 | 0.023 | 0.11 | 0.7406 |
| BC | 5.29 | 1 | 5.29 | 26.81 | 0.0001 |
| BD | 0.01 | 1 | 0.01 | 0.051 | 0.8251 |
| CD | 71.40 | 1 | 71.40 | 361.82 | <0.0001 |
| A2 | 23.81 | 1 | 23.81 | 120.64 | <0.0001 |
| B2 | 18.00 | 1 | 18.00 | 91.21 | <0.0001 |
| C2 | 348.35 | 1 | 348.35 | 1765.20 | <0.0001 |
| D2 | 23.50 | 1 | 23.50 | 119.07 | <0.0001 |
| 残差 | 2.76 | 14 | 0.20 | ||
| 失拟误差 | 2.33 | 10 | 0.23 | 2.16 | 0.2385 |
| 纯误差 | 0.43 | 4 | 0.11 | ||
| 总误差 | 664.47 | 28 | |||
| 标准偏差 | 0.44 | 预测系数R | 0.9788 | ||
| 确定系数R2 | 0.9958 | 变异系数 | 0.59% | ||
| 调整系数R | 0.9917 | 精密度 | 56.170 | ||
| 来源 | 平方和 | 自由度 | 均方 | F值 | P值 |
|---|---|---|---|---|---|
| 模型 | 661.71 | 14 | 47.26 | 239.50 | <0.0001 |
| A | 60.30 | 1 | 60.30 | 305.56 | <0.0001 |
| B | 57.64 | 1 | 57.64 | 292.08 | <0.0001 |
| C | 64.40 | 1 | 64.40 | 326.35 | <0.0001 |
| D | 36.75 | 1 | 36.75 | 186.22 | <0.0001 |
| AB | 5.52 | 1 | 5.52 | 27.98 | 0.0001 |
| AC | 8.70 | 1 | 8.70 | 44.10 | <0.0001 |
| AD | 0.023 | 1 | 0.023 | 0.11 | 0.7406 |
| BC | 5.29 | 1 | 5.29 | 26.81 | 0.0001 |
| BD | 0.01 | 1 | 0.01 | 0.051 | 0.8251 |
| CD | 71.40 | 1 | 71.40 | 361.82 | <0.0001 |
| A2 | 23.81 | 1 | 23.81 | 120.64 | <0.0001 |
| B2 | 18.00 | 1 | 18.00 | 91.21 | <0.0001 |
| C2 | 348.35 | 1 | 348.35 | 1765.20 | <0.0001 |
| D2 | 23.50 | 1 | 23.50 | 119.07 | <0.0001 |
| 残差 | 2.76 | 14 | 0.20 | ||
| 失拟误差 | 2.33 | 10 | 0.23 | 2.16 | 0.2385 |
| 纯误差 | 0.43 | 4 | 0.11 | ||
| 总误差 | 664.47 | 28 | |||
| 标准偏差 | 0.44 | 预测系数R | 0.9788 | ||
| 确定系数R2 | 0.9958 | 变异系数 | 0.59% | ||
| 调整系数R | 0.9917 | 精密度 | 56.170 | ||
| 反应器 | 单位体积换热面积/m2·m-3 | 年产量 /t | 产量放大 方式 | 放大效应 |
|---|---|---|---|---|
| AFRTM-G1 | 2500 | 25~80 | 数增放大 | 无放大效应 |
| AFRTM-G4 | 2500 | 约2000 | — | — |
| 半间歇反应釜 | 10 | 约1500 | 尺寸放大 | 小试-中试-生产 |
| 反应器 | 单位体积换热面积/m2·m-3 | 年产量 /t | 产量放大 方式 | 放大效应 |
|---|---|---|---|---|
| AFRTM-G1 | 2500 | 25~80 | 数增放大 | 无放大效应 |
| AFRTM-G4 | 2500 | 约2000 | — | — |
| 半间歇反应釜 | 10 | 约1500 | 尺寸放大 | 小试-中试-生产 |
| 1 | JIMENEZ-GONZALEZ C, POECHLAUER P, BROXTERMAN Q B, et al. Key green engineering research areas for sustainable manufacturing: a perspective from pharmaceutical and fine chemicals manufacturers[J]. Organic Process Research & Development, 2011, 15(4): 900-911. |
| 2 | LIM F P L, DOLZHENKO A V. Atom economy in green organic synthesis[M]//Green sustainable process for chemical and environmental engineering and science. Amsterdam: Elsevier, 2020: 1-12. |
| 3 | TONNE P, JAEDICKE H. Preparation of saccharin: US4464537[P]. 1984-08-07. |
| 4 | 齐枫楠, 杨国新, 赵利宽, 等.一种糖精钠的生产方法: CN103709118A[P]. 2014-04-09. |
| QI F N, YANG G X, ZHAO L K, et al. Method for producing sodium saccharin: CN103709118A[P]. 2014-04-09. | |
| 5 | GILLERON P, WLODARCZYK N, HOUSSIN R, et al. Design, synthesis and biological evaluation of substituted dioxodibenzothiazepines and dibenzocycloheptanes as farnesyltransferase inhibitors[J]. Bioorganic & Medicinal Chemistry Letters, 2007, 17(19): 5465-5471. |
| 6 | SHENG M, FRURIP D, GORMAN D. Reactive chemical hazards of diazonium salts[J]. Journal of Loss Prevention in the Process Industries, 2015, 38: 114-118. |
| 7 | SHUKLA C A, KULKARNI A A, RANADE V V. Selectivity engineering of the diazotization reaction in a continuous flow reactor[J]. Reaction Chemistry & Engineering, 2016, 1(4): 387-396. |
| 8 | KITTSLEY S. Need for minimum standards in evaluating doctoral programs[J]. Journal of Chemical Education, 1971, 48(6): 419. |
| 9 | ULLRICH R, GREWER T. Decomposition of aromatic diazonium compounds[J]. Thermochimica Acta, 1993, 225(2): 201-211. |
| 10 | PARTINGTON S, WALDRAM S P. Runaway reaction during production of an azo dye intermediate[J]. Process Safety and Environmental Protection, 2002, 80(1): 33-39. |
| 11 | 殷亘令, 李庆云. 从一起爆炸事故看DDNP生产设备的安全问题[J]. 爆破器材, 2000, 29(2): 28-30. |
| YIN G L, LI Q Y. The safety analysis of on explosion accident from the equipment used in the production of DDNP[J]. Explosive Materials, 2000, 29(2): 28-30. | |
| 12 | MARALLA Y, SONAWANE S, KASHINATH D, et al. Process intensification of tetrazole reaction through tritylation of 5-[4’-(methyl) biphenyl-2-Yl] using microreactors[J]. Chemical Engineering and Processing Process Intensification, 2017, 112: 9-17. |
| 13 | ZHANG F X, NOYERIE C C, WOEHI P, et al. Intensified liquid/liquid mass transfer in corning advanced-flow reactors[J]. Chemical Engineering Transactions, 2011, 24: 1369-1374. |
| 14 | 穆金霞,殷学锋. 微通道反应器在合成反应中的应用[J]. 化学进展, 2008, 20(1): 60-75. |
| MU J X, YIN X F. Application of microfluidic reactors on synthesis reactions[J]. Progress in Chemistry, 2008, 20(1): 60-75. | |
| 15 | GUTMANN B, CANTILLO D, KAPPE C O. Continuous-flow technology—A tool for the safe manufacturing of active pharmaceutical ingredients[J]. Angewandte Chemie International Edition, 2015, 54(23): 6688-6728. |
| 16 | MALET-SANZ L, MADRZAK J, HOLVEY R S, et al. A safe and reliable procedure for the iododeamination of aromatic and heteroaromatic amines in a continuous flow reactor[J]. Tetrahedron Letters, 2009, 50(52): 7263-7267. |
| 17 | USUTANI H, TOMIDA Y, NAGAKI A, et al. Generation and reactions of o-bromophenyllithium without benzyne formation using a microreactor[J]. Journal of the American Chemical Society, 2007, 129(11): 3046-3047. |
| 18 | DEADMAN B J, COLLINS S G, MAGUIRE A R. Taming hazardous chemistry in flow: the continuous processing of diazo and diazonium compounds[J]. Chemistry, 2015, 21(6): 2298-2308. |
| 19 | ECKERT H, AUERWECK J. Solvent-free and safe process for the quantitative production of phosgene from triphosgene by deactivated imino-based catalysts[J]. Organic Process Research & Development, 2010, 14(6): 1501-1505. |
| 20 | CANTILLO D, DAMM M, DALLINGER D, et al. Sequential nitration/hydrogenation protocol for the synthesis of triaminophloroglucinol: safe generation and use of an explosive intermediate under continuous-flow conditions[J]. Organic Process Research & Development, 2014, 18(11): 1360-1366. |
| 21 | WOOTTON R C, FORTT R, DE MELLO A J. On-chip generation and reaction of unstable intermediates-monolithic nanoreactors for diazonium chemistry: azo dyes[J]. Lab on a Chip, 2002, 2(1): 5-7. |
| 22 | PINHO V D, GUTMANN B, MIRANDA L S M, et al. Continuous flow synthesis of α-halo ketones: essential building blocks of antiretroviral agents[J]. The Journal of Organic Chemistry, 2014, 79(4): 1555-1562. |
| 23 | YU Z Q, LV Y W, YU C M, et al. Continuous flow reactor for Balz-Schiemann reaction: a new procedure for the preparation of aromatic fluorides[J]. Tetrahedron Letters, 2013, 54(10): 1261-1263. |
| 24 | QU T X, NIU S L, GONG Z Q, et al. Wollastonite decorated with calcium oxide as heterogeneous transesterification catalyst for biodiesel production: optimized by response surface methodology[J]. Renewable Energy, 2020, 159: 873-884. |
| 25 | SARAFRAZ M M, SAFAEI M R, GOODARZI M, et al. Experimental investigation and performance optimisation of a catalytic reforming micro-reactor using response surface methodology[J]. Energy Conversion and Management, 2019, 199: 111983. |
| 26 | 杨玉宇, 陈冰冰, 张元平. 无冷却螺旋管式苯胺重氮化反应工艺试验研究[J]. 浙江工业大学学报, 2009, 37(2): 213-216. |
| YANG Y Y, CHEN B B, ZHANG Y P. Experimental study on aniline diazotization without cooling in a spiral reactor[J]. Journal of Zhejiang University of Technology, 2009, 37(2): 213-216. | |
| 27 | STOESSEL F. Thermal safety of chemical processes: risk assessment and process design[M]. Germany: Wiley-VCH, 2008: 189-193. |
| 28 | ZOU Y, ZHANG T, WANG G, et al. Microfluidic continuous flow synthesis of 1,5-ditosyl-1,5-diazocane-3,7-dione using response surface methodology[J]. Journal of Industrial and Engineering Chemistry, 2020, 82: 113-121. |
| 29 | YUAN Y Z, ZHANG Y M, LIU T, et al. Optimization of microwave roasting-acid leaching process for vanadium extraction from shale via response surface methodology[J]. Journal of Cleaner Production, 2019, 234: 494-502. |
| 30 | 孙烁, 刘其友, 陈水泉, 等. 利用响应面法对L-2菌株降解石油烃进行优化[J]. 化工进展, 2019, 38(12): 5512-5518. |
| SUN S, LIU Q Y, CHEN S Q, et al. Optimization for degradation of total petroleum hydrocarbon by the strain L-2 with response surface methodology[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5512-5518. | |
| 31 | PARK Y J, YU T, YIM S J, et al. A 3D-printed flow distributor with uniform flow rate control for multi-stacked microfluidic systems[J]. Lab on a Chip, 2018, 18(8): 1250-1258. |
| 32 | WEI M, BOUTIN G, FAN Y, et al. Numerical and experimental investigation on the realization of target flow distribution among parallel mini-channels[J]. Chemical Engineering Research and Design, 2016, 113: 74-84. |
| 33 | Inc Corning. Devices for microreactor fluid distribution: EP2193839 (B1)[P]. 2019-03-13. |
| 34 | 马俊海. 康宁微通道反应器技术-强化传质传热,绿色连续合成[C]//中国化工学会橡塑产品绿色制造专业委员会微通道反应技术研讨和产业化推进会. 南京, 2016: 148-168. |
| MA J H. Corning microchannel reactor technology-enhanced mass and heat transfer, green continuous synthesis[C]//Rrubber and Plastic Rroducts Green Manufacturing Professional Committee, Microchannel Reaction Technology Seminar and Industrialization Promotion Meeting. Nanjing, 2016: 148-168. |
| [1] | CHANG Yinlong, ZHOU Qimin, WANG Qingyue, WANG Wenjun, LI Bogeng, LIU Pingwei. Research progress in high value chemical recycling of waste polyolefins [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3965-3978. |
| [2] | LIU Weixiao, LIU Yang, GAO Fulei, WANG Wei, WANG Yinglei. Application of microreactor in synthesis and quality improvement of energetic materials [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3349-3364. |
| [3] | WANG Zizong, LIU Gang, WANG Zhenwei. Progress and reflection on process intensification technology for ethylene/propylene production [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1669-1676. |
| [4] | XIAO Zhourong, LI Guozhu, WANG Li, ZHANG Xiangwen, GU Jianmin, WANG Desong. Research progress of the catalysts for hydrogen production via liquid hydrocarbon fuels steam reforming [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 97-107. |
| [5] | YAN Peng, CHENG Yi. Numerical simulation of membrane reactor of methane steam reforming for distributed hydrogen production [J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3446-3454. |
| [6] | YAN Lifang, CHU Bozhao, ZHONG Siqing, CHENG Yi. Synthesis of N-vinyl pyrrolidone by acetylene process in a microreactor [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 2902-2909. |
| [7] | JIANG Shengkun, HAN Bo, ZHAO Xin, YU Wanhe, LUO Guangsheng, DENG Jian, LIU Guangqi, WANG Jingqi, WANG Jinbo. Preparation of mononitrotoluene by continuous adiabatic nitration of excess toluene in microreactor [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 2910-2914. |
| [8] | SUN Xun, ZHAO Yue, XUAN Xiaoxu, ZHAO Shan, YOON Joon Yong, CHEN Songying. Advances in process intensification based on hydrodynamic cavitation [J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2243-2255. |
| [9] | SHI Yici, PAN Yanqiu, WANG Chengyu, FAN Jiahe, YU Lu. Experimental investigations on Joule effect enhanced air gap membrane distillation for water desalination [J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2285-2291. |
| [10] | SONG Fei, WANG Junyan, HE Lin, SUI Hong, LI Xingang. Surfactant enhancement of bubbling for separation of residual solvent from oil sands residue after solvent extraction [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2007-2014. |
| [11] | HAN Fen, YANG Na, SUN Yongli, JIANG Bin, XIAO Xiaoming, ZHANG Lyuhong. Removal of emulsified water in oil by glass fiber coalescer [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6723-6732. |
| [12] | WANG Deqiang, SUN Chao, WANG Kai, LUO Guangsheng. One-step continuous synthesis of anisole in microreactor [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6255-6260. |
| [13] | CHANG Tian, WANG Yu, ZHAO Zuotong, HU Jinchao, SHEN Zhenxing. Optimization of catalytic oxidation of trichloroethylene over Mn-Ce/HZSM-5 using response surface methodology [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5830-5842. |
| [14] | LENG Nanjiang, MA Guoguang, ZHANG Tao, LEI Yang, PENG Hao, XIONG Zuoshuai, CHEN Yuting. Research and exploration on purification of natural gas with high organic sulfur content [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5342-5353. |
| [15] | WANG Yanqian, WANG Yuanyang. Research progress of Fischer-Tropsch synthesis in microreactor [J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 185-191. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||